Open Access Article
Open Access ArticleA new concept for production of (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose, a precursor of D-fagomine†
Martina
Sudar
a,
Zvjezdana
Findrik
*a,
Đurđa
Vasić-Rački
a,
Anna
Soler
b and
Pere
Clapés
b
aUniversity of Zagreb, Faculty of Chemical Engineering and Technology, Savska c. 16, HR-10000 Zagreb, Croatia. E-mail: zfindrik@fkit.hr; Fax: +385 1 4597 133; Tel: +385 1 4597 157
bInstitute of Advanced Chemistry of Catalonia, Biotransformation and Bioactive Molecules Group, IQAC-CSIC, Jordi Girona 18-26, 08034 Barcelona, Spain
First published on 11th August 2015
Abstract
A novel cascade reaction for the production of aldol adduct (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose was studied in this work. The strategy combines three enzymes in one pot: (i) horse liver alcohol dehydrogenase for the oxidation of N-Cbz-3-aminopropanol to the corresponding aldehyde, (ii) NADH oxidase for the regeneration of coenzyme NAD+ and (iii) D-fructose-6-phosphate aldolase from E. coli A129S variant for the aldol addition of dihydroxyacetone to N-Cbz-3-aminopropanal. On the basis of preliminary experiments, optimization of the initial reaction conditions was done using statistical methods, i.e. factorial design of experiments. 79% yield of aldol adduct was achieved in the batch reactor after optimization.
Introduction
Multistep enzyme-catalyzed reactions1 are gaining significant attention, especially for the industrial production of fine chemicals in the pharmaceutical sector and of commodity bulk chemicals due to their low environmental impact and sustainability.2,3 One interesting approach to these reactions is the use of several different cell-free biocatalysts in one-pot (Systems Biocatalysis)4–8 mimicking the biosynthetic pathways in living cells.9 These reactions are simple to operate and analyze for pathway engineering and process optimization. There is no need for the isolation of reaction intermediates, which results in favorable yields and lower consumption of chemicals.4,9 Furthermore, unfavorable equilibrium reactions can also benefit from the cascade concept since continuous removal of product in a subsequent reaction step can drive the reaction towards product formation.6 Up to now, a large number of biocatalytic cascade reactions has been developed; from simple oxido-reduction reaction coupled with cofactor regeneration, to highly complex multi-enzyme synthesis.4,10In this work, a novel enzymatic cascade consisting of amino alcohol oxidation to amino aldehyde and aldol addition reaction was developed for the synthesis of the aldol precursor (3, Scheme 1) of D-fagomine. D-Fagomine is a naturally occurring iminosugar which can be found in plants in small quantities.11,12 It is a known inhibitor of glycoprocessing enzymes13–15 which reduces the risk of developing insulin resistance and obesity16 and may promote the adhesion of beneficial bacteria (e.g. Lactobacillus).17 It can be used as dietary supplement or a component of functional food.18 The synthesis of this compound via chemo-enzymatic pathway is a prime example of the efficiency of such method in comparison to purely chemical process.19
 | ||
| Scheme 1 Reaction scheme of cascade reaction of amino alcohol 1 oxidation to 2 with coenzyme regeneration using NOX and aldol addition of DHA to 2 catalyzed by FSA A129S. | ||
The strategy devised in this work consisted of the simultaneous use of three enzymes: horse liver alcohol dehydrogenase (HLADH) used for the oxidation of N-Cbz-3-aminopropanol (1) to N-Cbz-3-aminopropanal (2), D-fructose-6-phosphate aldolase (FSA) variant A129S for the aldol addition, and a third enzyme; NADH oxidase (NOX) for coenzyme regeneration (Scheme 1). The product of this cascade reaction is (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose (3, Scheme 1), a precursor of D-fagomine. The aldol addition of dihydroxyacetone (DHA) to 2 catalyzed by FSA to furnish 3, was studied in detail in our previous works.20,21 Since the amino aldehyde 2 is unstable, the purpose of the cascade reaction was to produce it in situ by oxidation of 1. Similar amino alcohol was oxidized by different authors in reactions catalyzed by chloroperoxidase with the addition of tert-butyl hydroperoxide or hydrogen peroxide,22 by laccase/O2/TEMPO23 and alcohol oxidase.23 These systems avoid the coenzyme addition, but all of them are unselective towards the substrate, and result in over-oxidation of the part of amino alcohol to amino acid. We propose the use of horse liver alcohol dehydrogenase, which was successfully tested for oxidation of different amino alcohols,24 and the use of NADH oxidase (NOX) for coenzyme regeneration. NOX is a flavoprotein which oxidizes NADH to NAD+, and was isolated and purified from Lactococcus lactis.25,26 To the best of our knowledge this kind of system (Scheme 1) was not investigated before. Amino alcohol oxidation combined with aldol addition was studied by Mifsud and co-authors23 in one pot – two step consecutive reactions because of the incompatible reaction conditions for both biocatalysts. In the present approach, the reaction was carried out in one-pot with simultaneous addition of three enzymes. This system is a highly promising alternative to the one previously mentioned, since coenzyme regeneration is elegantly resolved by the addition of NADH oxidase, and the problem of thermodynamic equilibrium of oxidation25,27,28 catalyzed by ADH could be overcome. The studied process uses environmentally friendly technology and offers a novel approach to the subject. It spares the enzymes of negative effect of peroxides used in previous works22,23 and of additional chemicals such as TEMPO which pollute the product and complicate its purification. Coenzyme regeneration by NADH oxidase does not add any new compounds to the system, and requires only a minimal amount of oxygen in the solution for the maximum enzyme activity.25
Materials and methods
Chemicals
NAD+, NADH and N-Cbz-3-aminopropanoic acid (4) were purchased from Acros Organics. N-Cbz-3-aminopropanol (1), DHA, acetonitrile, methanol, hydrochloric acid (HCl), triethanolamine (TEA), trifluoroacetic acid (TFA) and horse liver alcohol dehydrogenase (HLADH) were purchased from Sigma Aldrich (Germany). N-Cbz-3-aminopropanal (2) and (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose (3) were synthesized as previously described,15 in the labs of Biotransformation and Bioactive Molecules Group at Institute of Advanced Chemistry of Catalonia IQAC-CSIC (Spain). D-Fructose-6-phosphate aldolase from E. coli A129S variant was expressed and purified in the labs of IQAC-CSIC (Spain). NADH oxidase from Lactococcus lactis was isolated and purified in our laboratory according to the procedure described previously.25,26HPLC analysis
N-Cbz-3-aminopropanol (1), N-Cbz-3-aminopropanal (2), N-Cbz-3-aminopropanoic acid (4) and the aldol adduct (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose (3) concentrations were analysed using high performance liquid chromatography (HPLC) (Shimadzu, Japan) on a Lichrospher® (Phenomenex) 250 × 4 (5 μm) column at 30 °C and at 215 nm. Mobile phase consisted of mobile phase A (water and 0.1% TFA) and mobile phase B (80% acetonitrile, 20% water, 0.095% TFA). Gradient elution from 30 to 100% B during 30 minutes at total flow rate of 1.2 cm3 min−1 was used.29 The retention times for 3, 1, 4 and 2 were 4.6, 6.8, 7.4 and 7.7 min, respectively. External standard methodology was used for the quantification.Enzyme activity measurements
Enzyme activity was determined during the experiments in order to estimate the operational stability decay rate constant of the enzyme (data not shown). The samples were taken from the batch reactor at different time intervals.FSA activity measurements
FSA activity was determined by starting separate batch experiments with the samples taken from the reactor according to the previously described method.21 One unit of aldolase activity was defined as the amount of enzyme necessary to produce one μmol of 3 in one minute at 25 °C and in the selected buffer with 10% v/v of acetonitrile.HLADH activity measurements
Activity of HLADH was measured on spectrophotometer at 340 nm following the increase of NADH in the reaction of ethanol oxidation30 and oxidation of 1 for pH dependence of enzyme activity. One unit of HLADH was defined as the amount of enzyme necessary to oxidize 1 μmol of ethanol or 1 per minute at 25 °C and in the selected buffer with 10% v/v of acetonitrile.NADH oxidase activity measurements
NOX activity was followed via spectrophotometer by measuring the decrease of NADH in the reaction of NADH oxidation.31 One unit of NADH oxidase activity was defined as the amount of enzyme necessary to oxidize 1 μmol of NADH per minute at 25 °C and in the selected buffer.Batch reactor experiments
Cascade reaction of amino alcohol oxidation with coenzyme regeneration and aldol addition was carried out in a 1 mL batch reactor on an IKA Vibrax VXR basic shaker at 1000 rpm and at 25 °C. The reaction media was 50 mM TEA HCl buffer, pH 8.0, with 10% v/v of acetonitrile. The presence of an organic co-solvent is necessary due to the low solubility of amino alcohol and amino aldehyde in water. During the reaction, samples of 10 μL were taken from the reactor, dissolved in methanol and filtered (0.22 μm filter) for HPLC analysis. All experiments were done in duplicates.Protocols for purification of 3 and the NMR analysis were identical to those described in the literature.15
Reaction yield of the main product (3) and the by-product (4) were calculated according to eqn (1).
 | (1) |
Enzyme operational stability decay rate was described by the kinetics of the first order20 according to eqn (2).
 | (2) |
Enzyme operational stability decay rate constants were estimated by non-linear regression methods (simplex and least squares fit) implemented in SCIENTIST software32 using the obtained experimental data. They were estimated by fitting the model to the experimental data – the change in specific activity of enzyme during the experiment. The calculated data were compared with the experimental data, recalculated in the optimization routine and fitted again until a minimal error between experimental and integrated values was achieved. The residual was defined as the sum of squares of the differences between the experimental and calculated data.
Optimization of aldol adduct and amino acid yield in the aldol addition using the two-level factorial design
The statistical study of the process was performed using two-level full factorial experimental design with three process variables: NAD+, FSA and ADH concentrations. Development of the full 23 factorial experimental design with one central point was carried out using Design-Expert©, a DoE software from Stat-Ease, Inc. Experimental results were analyzed by applying ANOVA technique implemented in the Design-Expert© software. Operating variables and their levels are presented in Table 1. Concentrations of 1 (cN-Cbz-3-aminopropanol = 10.00 mM), DHA (cDHA = 25.00 mM) and NOX (γNOX = 3.25 mg mL−1, 0.07 U) were kept constant. Temperature (25 °C), pH (8.0) and reaction time (72 h) were constant in all the experiments as well. NOX concentration was kept constant and high in all experiments to ensure efficient coenzyme regeneration in all experiments and to compensate its activity loss. The concentration of DHA was kept higher than equimolar to ensure the equilibrium shift towards the aldol adduct in the aldol addition. The reaction was kept running for 72 hours to ensure its equilibrium conversion. The experiments were carried out in 0.1 mL reactor volume on IKA Vibrax shaker at 1000 rpm. 1 mg mL−1 of FSA corresponds to 2.31 U mL−1 of enzyme activity. 1 mg mL−1 of ADH corresponds to 0.003 U mL−1 of enzyme activity to 1.| Variable name | Unit | Lower limit | Center point | Upper limit |
|---|---|---|---|---|
| A: NAD+ | mM | 0.10 | 0.55 | 1.00 |
| B: FSA | mg mL−1 | 1.00 | 2.00 | 3.00 |
| C: HLADH | mg mL−1 | 1.00 | 5.50 | 10.00 |
The experimental design was composed of 18 experiments in total (23 + 1 central point in duplicates). The variable ranges were chosen according to the practical assumptions. If efficient regeneration system is present, coenzyme should not be used in the equimolar concentration in comparison to substrate concentration. That is why the maximum concentration was chosen to be only 1 mM. The lower concentration limit was chosen to be 100-fold lower than the concentration of 1. The concentrations of FSA and HLADH were chosen on the basis of preliminary experiments and previous experience which showed that higher increase of their concentration does not have significant influence on the process outcome at the proposed initial concentrations of 1, DHA, and NOX.
The concentration of 3 and 4 formed after 72 h were measured and used as the responses of the process. It was the purpose of the optimization to find the conditions at which maximum yield of 3 will be achieved, and possibly to minimize the over-oxidation of 1 to 4.
A factorial model is composed of a list of coefficients multiplied by an associated factor (investigated variable). It can be presented by the eqn (3), where the coefficients b are associated with a certain variable, designated as letters A, B and C representing the NAD+, FSA and HLADH concentration. Interactions of these variables are presented as the combination of letters.
| Response = b0 + b1A + b2B + b3C + b12AB + b13AC + b23BC + b123ABC | (3) |
The objective was to maximize the yield/concentration of 3 and minimize 4 in the investigated variable range.
Results and discussion
The choice of buffer and the optimal pH
For the cascade reaction with two primary enzymes (i.e. HLADH and FSA A129S) and one secondary enzyme (i.e. NOX), the buffer and optimal pH had to be chosen. To this end, the influence of pH on enzyme activity was investigated to find out the conditions at which all enzymes would be sufficiently active to carry out the biotransformation cascade. The compromise (Fig. 1) was found at 50 mM TEA HCl buffer, pH 8.0 that was further used as reaction media for the cascade reaction experiments. The three enzymes exhibited high practical catalytic activity at this pH. This is consistent with the optimal values found in the literature, i.e. pH 7.5 for oxidation and aldol reactions33 and pH 8.7, for amino alcohol oxidation catalyzed by horse liver ADH.24Cascade reaction
Cascade reaction was carried out in the batch reactor experiments at different initial reaction conditions (Table 2, Fig. 2). To successfully perform this kind of reaction with high reaction yield of 3, it is necessary to choose the appropriate initial reaction conditions, i.e. concentration of the three enzymes, coenzyme NAD+, 1 and DHA. At conditions of exp 1 (Table 2) the conversion was meager and only some trace concentration of 3 were observed, (Table 2, exp 1). In addition, 4 was formed as a consequence of over-oxidation, consistent with the results reported by other authors in less specific oxidation systems (Scheme 1).22,23 Under the conditions of exp 2 (Table 2) no 4 was formed probably due to the lower concentration of NAD+ used. The literature suggests that the over-oxidation can be catalyzed by alcohol dehydrogenase.36–38 This issue will be discussed further in detail.| Experiment | c NAD+/mM | c DHA/mM | γ NOX/mg mL−1 | Y aldol/% | Y amino acid/% |
|---|---|---|---|---|---|
| a c amino alcohol = 11.24 mM, γHLADH = 5.07 mg mL−1, 0.015 U, γFSA = 5.07 mg mL−1, 11.71 U. This experiment is not presented in Fig. 2. b c amino alcohol = 12.38 mM, γHLADH = 5.00 mg mL−1, 0.015 U, γFSA = 2.09 mg mL−1, 4.83 U. | |||||
| 1a | 1.00 | 10.02 | 0.12 | 11.73 | 21.89 |
| 2b | 0.10 | 25.23 | 0.45 | 32.18 | 0.00 |
| 3b | 1.00 | 10.00 | 0.45 | 31.09 | 23.56 |
| 4b | 1.00 | 25.23 | 0.18 | 53.89 | 22.27 |
| 5b | 1.02 | 25.23 | 0.45 | 75.71 | 15.07 |
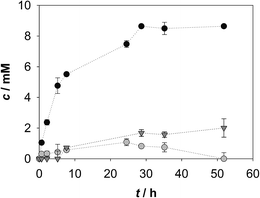
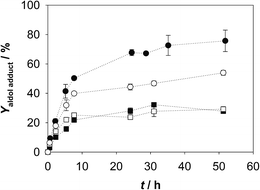 | ||
| Fig. 2 Aldol adduct formed in the cascade reaction of amino alcohol oxidation with coenzyme regeneration and aldol addition reaction (Vr = 1 mL, 50 mM TEA HCl buffer pH 8.0, 10% v/v acetonitrile, camino alcohol = 12.38 mM, γADH = 5.00 mg mL−1, 0.015 U, γFSA = 2.09 mg mL−1, 4.83 U, initial concentrations of other reactants and enzymes are presented in Table 1; ··■·· – experiment 2, ··□·· – experiment 3, ··○·· – experiment 4, ··●·· – experiment 5). | ||
Theoretical consideration based on our previous studies conducted with FSA A129S variant20,21 and NOX25 were made on process variables that may have crucial influence on the process outcome; i.e. coenzyme, NOX and DHA concentrations. It was assumed that the efficiency of coenzyme regeneration was very important for the oxido-reduction. The experiments presented in Fig. 2 showed that NAD+ and NOX influence the formation of 3, and by increasing their concentration, higher yield of 3 was achieved. Like oxido-reduction, aldol addition is an equilibrium reaction as well and concentration of DHA higher than equimolar was used to shift the equilibrium towards the aldol adduct. Since the reverse C–C bond-forming processes are often favored by thermodynamic relationships, product concentration might be increased by working at higher substrate concentrations20,34 or by increasing the concentration of one of the reactants.34
With the preliminary experiments presented in Table 2 it can be seen that up to 76% of 3 can be formed (Fig. 3) and a low concentration of 2 is actually present in the reaction media during the reaction. Thus, a positive result of increased DHA concentration can be observed. The results also show that oxidation and aldol addition were, in fact, operating simultaneously and the problem of the instability of 2 was effectively resolved. The synthesized 3 was characterized by 1H and 13C NMR analysis. The results are presented in appendix, Fig. 1 together with the HPLC analysis of the product in comparison to the standard (appendix, Fig. 2).
To eliminate the possibility of side reaction between DHA and HLADH and 3 and ADH, spectrophotometric assays were done. It was found that NADH and DHA induce a low activity of HLADH, but in the absence of NADH, the activity is not present. This is very important because DHA and NAD+ do not react in the HLADH catalyzed reaction. With an efficient NAD+ coenzyme regeneration, there is virtually no NADH in the reaction system, and all coenzyme is present in NAD+ form. 3 did not show any activity with HLADH which is also visible from the batch reactor experiments, i.e. from the fact that concentration of 3 remains constant after achieving maximum reaction yield.
Enzyme operational stability decay rate constants
Enzyme activity for the three enzymes was determined to estimate the enzyme operational stability decay rate constants (Table 3). The most stable enzyme in the reaction media was HLADH while the other two enzymes lose their activity at approximately the same rate. Using eqn (2) and operational stability decay rate constants (Table 3), enzyme half-life times (t1/2) were calculated (Table 3). Half-life time for HLADH was 86.6 h and the half-life times for NOX and FSA were 10.5 and 8.9 h, respectively. The calculated t1/2 show that only HLADH retains its activity during cascade reactions carried out in this work (maximum time 72 h) and that the other two enzymes lose their activity by the end of the reaction. This means that properties of the enzymes should be improved by some of the available techniques35,36 for the better use of the biocatalysts in this system.| Enzyme | k d/h−1 | t 1/2/h |
|---|---|---|
| HLADH | 0.008 ± 0.003 | 86.6 |
| NOX | 0.066 ± 0.015 | 10.5 |
| FSA A129S | 0.078 ± 0.004 | 8.9 |
The t1/2 for FSA is in very good agreement with our previous work,20 which was done in different organic co-solvent. The low organic co-solvent concentration used (up to 10% v/v) did not have a significant influence on aldolase activity.21 Furthermore, NOX is more stable in the reaction system than in the absence of substrate which is also in accordance with our previous findings.25
Experimental plan and response analysis
Since the preliminary data showed a great potential for the yield of 3, statistical optimization was done. Design Expert software was used for that purpose. A series of experiments was carried out. The results and the design matrix are presented in Table 4. They show good repeatability. The maximum yield of 3 that was achieved in these experiments was 78.5% (mean value of experiments 2 and 10). The yield of 4 under the same conditions was 9.9% (mean value of experiments 2 and 10).| Run | A: cNAD+/mM | B: γFSA/mg mL−1 | C: γHLADH/mg mL−1 | Response 1 caldol adduct/mM | Response 1 camino acid/mM | Y aldol adduct/% | Y amino acid/% |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 1 | 1 | 1.14 | 0.06 | 11.4 | 0.6 |
| 2 | 1 | 3 | 10 | 7.78 | 0.92 | 77.8 | 9.2 |
| 3 | 0.1 | 3 | 1 | 0.89 | 0.07 | 8.9 | 0.7 |
| 4 | 1 | 1 | 1 | 6.78 | 0.15 | 67.8 | 1.5 |
| 5 | 0.1 | 1 | 10 | 3.14 | 0.05 | 31.4 | 0.5 |
| 6 | 1 | 3 | 1 | 6.45 | 0.07 | 64.5 | 0.7 |
| 7 | 0.55 | 2 | 5.5 | 6.06 | 0.11 | 60.6 | 1.1 |
| 8 | 0.1 | 3 | 10 | 2.84 | 0.01 | 28.4 | 0.1 |
| 9 | 0.1 | 1 | 10 | 3.37 | 0.05 | 33.7 | 0.5 |
| 10 | 1 | 3 | 10 | 7.91 | 1.06 | 79.1 | 10.6 |
| 11 | 0.1 | 3 | 1 | 0.81 | 0.06 | 8.1 | 0.6 |
| 12 | 1 | 1 | 1 | 6.48 | 0.16 | 64.8 | 1.6 |
| 13 | 0.1 | 1 | 1 | 0.92 | 0.04 | 9.2 | 0.4 |
| 14 | 1 | 1 | 10 | 7.35 | 2.37 | 73.5 | 23.7 |
| 15 | 0.1 | 3 | 10 | 2.91 | 0.05 | 29.1 | 0.5 |
| 16 | 1 | 3 | 1 | 6.26 | 0.11 | 62.6 | 1.1 |
| 17 | 1 | 1 | 10 | 6.38 | 1.52 | 63.8 | 15.2 |
| 18 | 0.55 | 2 | 5.5 | 6.28 | 0.13 | 62.8 | 1.3 |
Statistical analysis37 of the results (Table 4) was done and the effect of each variable or their interaction on the objective function was determined. The calculations were done by the software and the results are presented in Table 5. P values show that concentrations of coenzyme (A) and HLADH (C), as well as interactions between NAD+ and FSA (AB), and NAD+ and HLADH (AC) concentrations are significant process variables in the investigated variable range. They have a significant influence on the equilibrium concentration of 3. Table 5 also implies that all single variables, i.e. NAD+ (A), FSA (B) and HLADH (C) concentrations have significant influence on the formation of 4 in the reaction mixture in the investigated variable range. Additionally, interactions between NAD+ and FSA (AB), and NAD+ and HLADH (AC) concentrations also have significant influence on this optimization objective.
| Source | Sum of squares | Degrees of freedom | Mean square | F value | P value |
|---|---|---|---|---|---|
| Aldol adduct concentration | |||||
| Model | 7.79134 | 7 | 1.113049 | 339.6448 | <0.0001 |
| A | 6.458416 | 1 | 6.458416 | 1970.774 | <0.0001 |
| B | 0.001212 | 1 | 0.001212 | 0.369977 | 0.5581 |
| C | 0.891378 | 1 | 0.891378 | 272.0024 | <0.0001 |
| AB | 0.027157 | 1 | 0.027157 | 8.286876 | 0.0182 |
| AC | 0.385004 | 1 | 0.385004 | 117.4832 | <0.0001 |
| BC | 0.012195 | 1 | 0.012195 | 3.72132 | 0.0858 |
| ABC | 0.015977 | 1 | 0.015977 | 4.875385 | 0.0546 |
| Curvature | 0.427986 | 1 | 0.427986 | 130.5991 | <0.0001 |
| Pure error | 0.029494 | 9 | 0.003277 | ||
| Cor total | 8.24882 | 17 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Amino acid concentration | |||||
| Model | 29.29351 | 7 | 4.184787 | 114.5687 | <0.0001 |
| A | 16.57768 | 1 | 16.57768 | 453.8544 | <0.0001 |
| B | 0.246378 | 1 | 0.246378 | 6.745198 | 0.0289 |
| C | 5.134697 | 1 | 5.134697 | 140.5748 | <0.0001 |
| AB | 0.472917 | 1 | 0.472917 | 12.94726 | 0.0058 |
| AC | 6.837377 | 1 | 6.837377 | 187.1898 | <0.0001 |
| BC | 0.023531 | 1 | 0.023531 | 0.644217 | 0.4429 |
| ABC | 0.000922 | 1 | 0.000922 | 0.025252 | 0.8772 |
| Curvature | 0.068345 | 1 | 0.068345 | 1.871101 | 0.2045 |
| Pure error | 0.328738 | 9 | 0.036526 | ||
| Cor total | 29.69059 | 17 | |||
Multiple regression analysis of experimental data resulted in model eqn (4) and (5), which describe the influence of each investigated variable on the objective functions. Their coefficients are given as coded values.
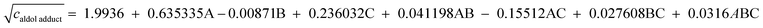 | (4) |
| ln(camino acid) = −1.92465 + 1.017893A − 0.12409B + 0.566497C − 0.17192AB + 0.653709AC − 0.03835BC + 0.007593ABC | (5) |
The model adequacy was checked by ANOVA analysis. The model F values for 3 and 4 concentration objectives were 339.64 and 114.57, respectively. This means that both models are significant. There is only a 0.01% chance that F values this large could occur due to noise in both cases. ANOVA results are summarized in Table 5. The model adequacy was tested by the residual analysis (appendix, Fig. 3), residuals vs. predicted plot (appendix, Fig. 4), predicted vs. actual plot (appendix, Fig. 5) etc. R2 values for 3 and 4 concentrations as objective functions were found to be 0.996 and 0.989, respectively.
 | ||
| Fig. 4 Graphical interpretations of the model (eqn (4)) for aldol adduct concentration. The influence of coenzyme ((a) cNAD+ = 1.00 mM, (b) cNAD+ = 0.10 mM), HLADH ((c) γADH = 10.00 mg mL−1, (d) γADH = 1.00 mg mL−1) and FSA ((e) γFSA = 3.00 mg mL−1, (f) γFSA = 1.00 mg mL−1) concentration on the aldol adduct concentration. | ||
 | ||
| Fig. 5 Graphical interpretations of the model (eqn (5)) for amino acid concentration. The influence of coenzyme ((a) cNAD+ = 1.00 mM, (b) cNAD+ = 0.10 mM), HLADH ((c) γADH = 10.00 mg mL−1, (d) γADH = 1.00 mg mL−1) and FSA ((e) γFSA = 3.00 mg mL−1, (f) γFSA = 1.00 mg mL−1) concentration on the amino acid concentration. | ||
The model eqn (4), as well as its graphical interpretation presented in Fig. 4, suggests that higher coenzyme (A) and HLADH (C) concentration result in higher concentration of 3. The model eqn (5), as well as its graphical interpretation presented in Fig. 5 show that lower content of 4 would be obtained in the reaction mixture if concentrations of coenzyme (A) and HLADH (C) were decreased. Additionally, higher concentrations of FSA favor the formation of lower concentrations of 4 (Fig. 5c and d) which is due to faster consumption of 2.
It can be seen from the simulations (Fig. 4) how concentrations of HLADH, FSA and NAD+ influence the concentration of 3 (Fig. 4a–d). Fig. 5e–f show that concentration of 4 increases, as the concentration of NAD+ increases. This was suggested by our preliminary experiments. Additionally, concentration of 4 will increase at higher concentration of HLADH (Fig. 5e–f) which implies that over-oxidation of 1 to 4 might be catalyzed by HLADH. This was supported by literature38–40 which reports the ability of many ADHs to catalyze the oxidation of amino aldehyde to amino acid (in the presence of NAD(P)+), especially at higher pH values which favor the oxidation reaction. We conducted additional experiments with HLADH from a different supplier (prepared and purified by prof. Martina Pohl's research group, Research Center Jülich), and it was found that 4 also forms, which further confirms this hypothesis. Considering that this statistical analysis showed that both HLADH and NAD+ concentrations should be increased to produce more 4, it is an additional corroboration to this hypothesis.
Tables 1 and 2 in appendix present the optimal solutions for the separate objective functions calculated by the software. Since similar conditions regarding the concentrations of coenzyme and HLADH favor both the formation of 3 and 4, it was not desirable to join these two objectives into one optimization. This optimization would have been a compromise and would result in lower concentration of 3. Additionally, maximum yield of 3 was 79% (Table 4, exp 10) which is a good result. Hence, the results obtained in this work represent a remarkable achievement in enzymatic cascade oxidation–aldol reactions. For comparison, in a one-pot two-step cascade reaction system with oxidation-C–C bond formation using an analogous N-Cbz-amino alcohol, Mifsud and coauthors23 obtained 19% of aldol adduct and 81% yield of N-Cbz-amino acid in aqueous buffered media.
Conclusion
In this work, a novel cascade reaction consisting of three enzymes operating simultaneously in one-pot was successfully developed to produce the aldol adduct (3S,4R)-6-[(benzyloxycarbonyl)amino]-5,6-dideoxyhex-2-ulose (3), precursor of the iminocyclitol D-fagomine in good yields (79%). ADH from horse liver was used for the oxidation of 1 to produce 2 which is concomitantly used, i.e. in the same pot, as a substrate in the aldol addition catalyzed by FSA A129S to produce 3. In this reaction, NOX from L. lactis was successfully applied as a regenerating enzyme for the in situ regeneration of coenzyme NAD+. The use of a regeneration system is essential to make a process economically feasible. This cascade presents an interesting way to conduct aldol addition reactions using stable and readily available starting materials such as amino alcohols. Because of high instability of 2, as well as 1, used as the main reactant in this cascade, it would not be possible to carry out successfully this cascade reaction without the protecting group.Optimization of initial conditions of cascade reaction showed that higher concentrations of coenzyme and HLADH favor both the formation of 3 and 4. Maximal yield of 3 achieved in this cascade reaction was 79%. At the same time yield of 4 was 10%. The system might be further improved by finding the appropriate ADH which would not catalyze the over-oxidation of amino aldehyde to amino acid.
List of symbols and abbreviations
| c | Molar concentration, mM |
| k d | Operational stability decay rate constant, h−1 |
| S.A. | Specific activity, U mg−1 |
| T | Reaction time, min |
| t 1/2 | Half-life time, h |
| V.A. | Volume activity, U mL−1 |
| V enz | Enzyme volume, mL |
| V r | Reactor volume, mL |
| Y | Reaction yield, % |
| γ | Mass concentration, mg mL−1 |
| HLADH | Horse liver alcohol dehydrogenase |
| DHA | Dihydroxyacetone |
| FSA | D-Fructose-6-phosphate aldolase |
| HCl | Hydrochloric acid |
| NOX | NADH oxidase |
| TEA | Triethanolamine |
| TFA | Trifluoroacetic acid |
Acknowledgements
This work was supported by the Croatian Ministry of Science Education and Sports and Spanish National Research Council in the Frame of the Project ERA-IB: Microreactor technology for continuous enzymatic reactions catalyzed by C–C-bond forming enzymes (EIB. 10.012. MicroTechEnz, http://www.fkit.unizg.hr/miten) and by the Spanish MICINN CTQ2012-31605, Generalitat de Catalunya (2009 SGR 00281) and ERA-IB MICINN. PIM2010EEI-00607. Authors would also like to acknowledge the support from COST action: CM0701 and the new COST action CM1303 Systems Biocatalysis, the Croatian Ministry of Science Education and Sports for PhD scholarship of Martina Sudar and Susana Amezqueta from Bioglane S.L.N.E. for the assessment of purity of the Bioglane S.L.N.E. materials used in this work. The help of Apl. Prof. Dr Martina Pohl in providing HLADH purified in the lab of Research Centre Jülich is greatly acknowledged.References
- Z. Findrik and Đ. Vasić-Rački, Chem. Biochem. Eng. Q., 2009, 23, 545–553 CAS.
- R. Xue and J. M. Woodley, Bioresour. Technol., 2012, 115, 183–195 CrossRef CAS PubMed.
- Y. Ni, D. Holtmann and F. Hollmann, ChemCatChem, 2014, 6, 930–943 CrossRef CAS.
- W.-D. Fessner, New Biotechnol., 2015 DOI:10.1016/j.nbt.2014.11.007.
- Z. Findrik and Ð. Vasić-Rački, Biotechnol. Bioeng., 2007, 98, 956–967 CrossRef CAS PubMed.
- D. Monti, E. E. Ferrandi, I. Zanellato, L. Hua, F. Polentini, G. Carrea and S. Riva, Adv. Synth. Catal., 2009, 351, 1303–1311 CrossRef CAS.
- J. H. Sattler, M. Fuchs, F. G. Mutti, B. Grischek, P. Engel, J. Pfeffer, J. M. Woodley and W. Kroutil, Angew. Chem., Int. Ed., 2014, 53, 14153–14157 CrossRef CAS PubMed.
- http://www.cost.eu/COST_Actions/cmst/Actions/CM1303 .
- V. B. Urlacher and S. Schulz, in Cascade Biocatalysis, ed. S. Riva and W. D. Fessner, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2014, ch. 5, pp. 87–132 Search PubMed.
- E. Ricca, B. Brucher and J. H. Schrittwieser, Adv. Synth. Catal., 2011, 353, 2239–2262 CrossRef CAS.
- N. Asano, R. J. Nash, R. J. Molyneux and G. W. J. Fleet, Tetrahedron: Asymmetry, 2000, 11, 1645–1680 CrossRef CAS.
- M. Koyama and S. Sakamura, Agric. Biol. Chem., 1974, 38, 1111–1112 CrossRef CAS.
- R. J. Nash, A. Kato, C. Y. Yu and G. W. Fleet, Future Med. Chem., 2011, 3, 1513–1521 CrossRef CAS PubMed.
- M. Sugiyama, Z. Hong, P. H. Liang, S. M. Dean, L. J. Whalen, W. A. Greenberg and C. H. Wong, J. Am. Chem. Soc., 2007, 129, 14811–14817 CrossRef CAS PubMed.
- J. A. Castillo, J. Calveras, J. Casas, M. Mitjans, M. P. Vinardell, T. Parella, T. Inoue, G. A. Sprenger, J. Joglar and P. Clapés, Org. Lett., 2006, 8, 6067–6070 CrossRef CAS PubMed.
- S. Amézqueta, E. Galán, I. Vila-Fernández, S. Pumarola, M. Carrascal, J. Abian, L. Ribas-Barba, L. Serra-Majem and J. L. Torres, Food Chem., 2013, 136, 1316–1321 CrossRef PubMed.
- L. Gómez, E. Molinar-Toribio, M. A. Calvo-Torras, C. Adelantado, M. E. Juan, J. M. Planas, X. Cańas, C. Lozano, S. Pumarola, P. Clapés and J. L. Torres, Br. J. Nutr., 2012, 107, 1739–1746 CrossRef PubMed.
- T. K. Lim, in Edible medicinal and non-medicinal plants: Volume 5, Fruits, Springer Science+Business Media Dordrecht, 2013, pp. 459–493 Search PubMed.
- J. H. Schrittwieser and V. Resch, RSC Adv., 2013, 3, 17602–17632 RSC.
- M. Sudar, Z. Findrik, Ð. Vasić-Rački, P. Clapés and C. Lozano, J. Biotechnol., 2013, 167, 191–200 CrossRef CAS PubMed.
- M. Sudar, Z. Findrik, Ð. Vasić-Rački, P. Clapés and C. Lozano, Enzyme Microb. Technol., 2013, 53, 38–45 CrossRef CAS PubMed.
- M. Pešić, C. López and G. Álvaro, Biochem. Eng. J., 2012, 67, 218–224 CrossRef.
- M. Mifsud, A. Szekrenyi, J. Joglar and P. Clapés, J. Mol. Catal. B: Enzym., 2012, 84, 102–107 CrossRef CAS.
- L. Andersson and R. Wolfenden, Anal. Biochem., 1982, 124, 150–157 CrossRef CAS PubMed.
- M. Sudar, Z. Findrik, M. Vuković Domanovac and Ð. Vasić-Rački, Biochem. Eng. J., 2014, 88, 12–18 CrossRef CAS.
- F. Lopez de Felipe and J. Hugenholtz, Int. Dairy J., 2001, 11, 37–44 CrossRef CAS.
- A. Vrsalović Presečki and Đ. Vasić-Rački, Process Biochem., 2009, 44, 54–61 CrossRef.
- Z. Findrik, I. Šimunović and Ð. Vasić-Rački, Biochem. Eng. J., 2008, 39, 319–327 CrossRef CAS.
- A. L. Concia, C. Lozano, J. A. Castillo, T. Parella, J. Joglar and P. Clapés, Chem.–Eur. J., 2009, 15, 3808–3816 CrossRef CAS PubMed.
- Biochemica information II, Boehringer Mannheim GmbH, Biochemica, Germany, 1975, pp. 25–26 Search PubMed.
- B. R. Riebel, P. R. Gibbs, W. B. Wellborn and A. S. Bommarius, Adv. Synth. Catal., 2003, 345, 707–712 CrossRef CAS.
- SCIENTIST handbook, Micromath®, Salt Lake City, 1986-1995 Search PubMed.
- R. Schoevaart, F. van Rantwijk and R. A. Sheldon, J. Org. Chem., 2000, 65, 6940–6943 CrossRef CAS PubMed.
- W.-D. Fessner, in Modern aldol reactions Vol. 1: Enolates, organocatalysis, biocatalysis and natural product synthesis, ed. R. Mahrwald, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004, ch. 5, pp. 206–207 Search PubMed.
- J. M. Guisan, Immobilization of enzymes and cells, Humana Press, Totowa, New Jersey, 2nd edn, 2006 Search PubMed.
- K. B. Otte and B. Hauer, Curr. Opin. Biotechnol., 2015, 35, 16–22 CrossRef CAS PubMed.
- D. C. Montgomery, Design and analysis of experiments, Wiley, New York, 2001 Search PubMed.
- P. Könst, H. Merkens, S. Kara, S. Kochius, A. Vogel, R. Zuhse, D. Holtmann, I. W. C. E. Arends and F. Hollmann, Angew. Chem., Int. Ed., 2012, 51, 1–5 CrossRef.
- G. T. M. Henehan and N. J. Oppenheimer, Biochemistry, 1993, 32(3), 735–738 CrossRef CAS PubMed.
- A. Schmid, F. Hollmann and B. Buhler, in Enzyme Catalysis in Organic Synthesis: A Comprehensive Handbook, ed. K. Drauz and H. Waldmann, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2nd edn, 2008, ch 16.4, pp. 1194–1196 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14414k |
| This journal is © The Royal Society of Chemistry 2015 |