Electrosynthesis of a polyaniline/zeolite nanocomposite coating on copper in a three-step process and the effect of current density on its corrosion protection performance
Abstract
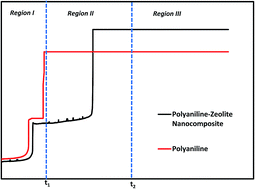
A polyaniline/zeolite nanocomposite (PZN) was successfully electrosynthesized at a copper (Cu) electrode in a sodium oxalate solution to generate a homogeneous and adherent coating. During the formation of the PZN coatings, three stages (electro-adsorption of monomer and electrolyte and initiation of formation of the passive film, growth and impingement of the passive film and decomposition of the latter and formation of composite coatings) are observed and three times related to these stages are reported. The coatings were characterized by SEM, XRD, FTIR and UV-vis spectroscopy. The corrosion behavior of copper with the PZN coatings in 3.5 wt% NaCl solution was investigated by potentiodynamic polarization and electrochemical impedance spectroscopy techniques. The effect of the applied current density on the protective properties of the PZN coatings has been investigated, and it was shown that the protection efficiency depends on the applied current density. The corrosion current density decreased from 43.56 μA cm−2 for un-coated copper to 0.34 μA cm−2 for PZN-coated copper under optimal conditions. It was observed that the PZN coating provided efficient protection (99.2%) to copper in 3.5 wt% NaCl solution. The corrosion rate for the PZN-coated copper was significantly lower than the bare copper (about 130 times).

 Please wait while we load your content...
Please wait while we load your content...