Microparticle-enhanced production of ε-poly-L-lysine in fed-batch fermentation†
Xi-Dong
Ren
,
Yong-Jie
Xu
,
Xin
Zeng
,
Xu-Sheng
Chen
*,
Lei
Tang
and
Zhong-Gui
Mao
*
The Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, 1800 Lihu Road, Wuxi 214122, Jiangsu, China. E-mail: chenxs@jiangnan.edu.cn; maozg@jiangnan.edu.cn; Fax: +86 510 85918296; Tel: +86 510 85918296
First published on 15th September 2015
Abstract
In this study, the effects of talc microparticles on mycelia morphology and ε-poly-L-lysine (ε-PL) production by Streptomyces sp. M-Z18 were investigated for the first time. Changing mycelia morphology with talc microparticles has resulted in ε-PL production being enhanced. Talc microparticles caused a decrease of pellet diameter from 297.63 ± 156.33 to 205.65 ± 71.56 μm in shake-flask fermentation. Moreover, the maximum ε-PL production reached 2.51 ± 0.08 g L−1 in the presence of 10 g L−1 talc microparticles, which was increased by 50.30%, compared to the control (1.67 ± 0.11 g L−1). The scale-up processes were performed in batch and fed-batch fermentations in a 5 L fermenter with 10 g L−1 talc addition, and the final ε-PL production of 30.57 g L−1 was obtained at 168 h, with 44.06% increase over the control. Talc microparticles of 10 g L−1 were then supplied to the fed-batch fermentation with acidic pH shock and agro-industrial by-products, and ε-PL production was further increased to 62.36 g L−1 at 192 h, compared to 54.70 g L−1 of the control without supplementary talc. This is also the highest ε-PL production reported so far in the literature. These results suggested that talc addition could be an efficient and universal approach for enhancing ε-PL production in industry.
1. Introduction
Filamentous bacteria such as Streptomyces exhibit diverse morphology in submerged cultivation, ranging from dispersed mycelia to dense pellets.1 Morphology is dependent on strain physiology, culture conditions and process parameters.2 Furthermore, production performance and morphology have been observed to be closely related.1,3,4 Due to this, many attempts have been stimulated to influence and control the morphology. To exemplify, Singh et al.4 have investigated the effects of phosphate and ammonia on the pellet morphology of Amycolatopsis balhimycina DSM 5908, and found that higher balhimycin productivity is correlated with higher pellet fraction in the biomass, small elongated pellets, and shorter filaments in hyphal growth in the periphery of the pellets. Besides, the addition of surfactant compounds was proved to influence morphology and formation of geldanamycin by Streptomyces hygroscopicus in a positive way.1 Most strikingly, a breakthrough in the targeted control of fungal morphology by inorganic microparticles (talc, aluminum oxide and titanium silicate oxide) was recently developed.5,6 This novel strategy allows the control of morphology only by physically disrupting spore aggregation, thus has minimum effects on other key parameters, which makes it ideal to study the relationship between morphology and product formation. However, little work has been done on morphology control of filamentous bacteria by inorganic microparticles.The filamentous bacteria Streptomyces sp. M-Z18 is a high-yield producer of ε-PL.7 The ε-PL, a natural homopolymer of amino acid, consisting of 25–35 L-lysine residues, is linked by the isopeptide bonds between α-carboxyl and ε-amino groups.8 Owing to its unique structure, ε-PL has been widely used as a natural food preservative in Japan, South Korea, United States and China.7,9 Moreover, ε-PL and its derivatives have also obtained wide spread application in the fields of food, medicine, environment and electronics.10 To date, ε-PL production is mainly based on microbial fermentation by Streptomyces. Fermentation process regulation, including medium optimization, pH control, immobilized cells and in situ product removal, has been successfully employed for the enhancement of ε-PL production.7,11–15 However, these approaches did not take into account the mycelia morphology itself as a potentially limiting factor for ε-PL production.
In this study, the effects of talc microparticles on morphology, cell growth and ε-PL production were first evaluated in shake-flask fermentation. Utilizing the optimum talc concentration, the processes were then transferred to batch and fed-batch fermentations in bioreactor. The results were compared with those obtained without talc addition. Finally, the influence of talc addition on ε-PL production was also considered in the fed-batch fermentation with acidic pH shock. Acidic pH shock with agro-industrial by-products utilization has been successfully developed to induce the overproduction of ε-PL in our previous study.7 The present work would provide useful information for the morphology control of filamentous bacteria by inorganic microparticles.
2. Materials and methods
2.1 Microorganism
Streptomyces sp. M-Z18 was used throughout this study, which was a mutagenesis from Streptomyces albulus Z-18 (CGMCC 10479).2.2 Culture media and inoculum preparation
Agar slant medium, used to maintain the strain, composed of (g L−1): glucose, 10; yeast extract, 5; beef extract, 5; MgSO4·7H2O, 0.5; K2HPO4·3H2O, 1; and agar 20, along with pH 7.0 before sterilization. Seed culture medium (M3G), contained (g L−1): glucose, 50; yeast extract, 5; (NH4)2SO4, 10; KH2PO4, 1.36; K2HPO4·3H2O, 0.8; MgSO4·7H2O, 0.5; ZnSO4·7H2O, 0.04; FeSO4·7H2O, 0.03. Fermentation medium containing (g L−1): glycerol, 60; (NH4)2SO4, 5; beef extract, 10; KH2PO4, 4; MgSO4·7H2O 0.8; FeSO4·7H2O, 0.05.15 The fermentation medium employing agro-industrial by-products, consisted of (g L−1): glycerol, 83; (NH4)2SO4, 8; fish meal, 15; corn steep liquor, 5; KH2PO4, 5; MgSO4·7H2O, 2; FeSO4·7H2O, 0.1,16 which was only used in the Section 3.5. Initial pH values of the above three media were adjusted to 6.8 with 2 M NaOH and/or 1 M H2SO4. All the media were sterilized in an autoclave for 20 min at 121 °C. In each case, glucose was autoclaved separately. The slants were inoculated and incubated at 30 °C for 7 days to obtain a heavy sporulated growth. After that time, spores were used for seed-culture inoculation (in a concentration of about 2 × 105 spores per mL). The seed culture was grown in a 500 mL Erlenmeyer flask containing 80 mL of liquid medium and incubated at 30 °C on a rotary shaker (200 rpm) for 24 h.2.3 Cultivation
Shake-flask fermentation was carried out in a 250 mL Erlenmeyer flask with 40 mL liquid fermentation medium. The fermentation medium was inoculated with 3 mL of a 24 h Streptomyces sp. M-Z18 seed-culture containing approximately 3 g L−1 dry cells and then cultured at 30 °C with a rotary velocity of 200 rpm for 72 h. Additionally, a 5 L fermenter (BIOTECH-5BG, BaoXing Bio-Engineering Equipment Co., Ltd, Shanghai, China) with a 3.5 L working volume and two Rushton turbines was employed for batch and fed-batch fermentations in this study. Before the inoculation, temperature, aeration rate and agitation speed were maintained at 30 °C, 0.5 vvm and 200 rpm, respectively, and initial pH was controlled at 6.8 via manual addition of ammonia water (12.5%, w/v). Approximately 300 mL of seed culture was used as the inoculum. Dissolved oxygen (DO) was set above 30% of air saturation before pH declined to 4.0 and above 20% of air saturation afterwards, which was controlled by manually adjusting agitation speed by the step of 50 rpm from 200 to 800 rpm during fermentation course. When agitation speed reached 800 rpm, aeration rate was then manually increased by the step of 0.5 vvm with a range of 0.5–2.5 vvm. During the fermentation, pH and DO were respectively monitored online by pH and DO electrodes (K8S-225 and InPro6800, Mettler Toledo, Greifensee, Switzerland), while ε-PL production, dry cell weights (DCW), glycerol, and ammonia nitrogen (NH4+-N) were analyzed offline as described in the Section 2.5. Fed-batch fermentation started when glycerol concentration in the fermentation broth was below 10 g L−1, and then sterilized pure glycerol was automatically added by peristaltic pump to maintain it at about 10 g L−1. Residual NH4+-N was maintained at about 0.5 g L−1 by feeding 600 g L−1 sterilized (NH4)2SO4 solution just as the feeding of glycerol. In all cultivations with talc addition, the microparticles with median diameter (D50) ∼ 6 μm (Guangyuan superfine powder Co., Ltd, Jiangyin, China) were added into both the seed culture and fermentation media at the beginning.2.4 Image analysis
The drawn samples were directly diluted with saline (0.9% NaCl) to about 105–106 pellets per mL. Then, twenty microliter samples were deposited on a clean slide and covered with a cover slip. Images of mycelia were captured by a CCD camera (Leica DFC 450) mounted on a microscope (Leica DM 1000). Morphological measurements were carried out using the Leica Application Suite V4 software (all from Leica Microsystems, Wetzlar, Germany). Measurements of mycelia morphology were done on images obtained using 4× objective. Distributions were generated with a population size of approximately 150 pellets per sample. The size of the pellet was estimated in terms of mean pellet diameter, while pellet shape was quantified as a ratio of the major to minor axis.2.5 Analytical methods
Ten millilitres of culture broth was subjected to centrifugation at 4500 × g for 10 min, and then the precipitate was collected and washed twice with distilled water. The washed mycelia were filtered through a pre-weighed filter paper and dried at 105 °C to a constant weight prior to measuring the DCW of the culture. All measurements were corrected for the microparticle added and were performed in triplicate. The supernatant was used to determine the ε-PL concentration according to the procedure described by Itzhaki.17 The concentration of glycerol was determined using an HPLC system (DIONEX, U-3000, USA) with a refractive index detector (Shodex RI-101, Japan) and an ion exchange column (Aminex HPX-87H, 300 × 7.8 mm, Hercules, CA). The column was eluted with 5 mM H2SO4 at a temperature of 60 °C and a flow rate of 0.6 mL min−1. NH4+-N was analyzed by means of a colorimetric method using Nessler reagent.18 To check the reproducibility, the experiments were carried out at least duplicate.2.6 Statistical analysis
The significance of samples was determined by analysis of variance, and sample means were separated by the student paired t test (p ≤ 0.05).3. Results and discussion
3.1 Influence of talc microparticles on mycelia morphology in shake-flask fermentation
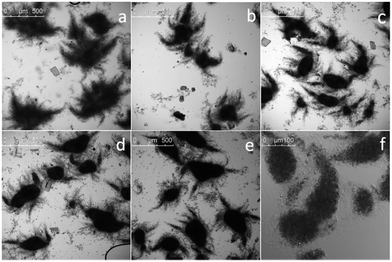
Adding talc into the culture showed significant impact on mycelia morphology (Fig. 1). Without talc addition, pellets were large, with a large number of peripheral mycelia (Fig. 1a). In the presence of talc, pellets became smaller, and the peripheral mycelia decreased with the incremental addition of talc microparticles (Fig. 1b–e). Notably, talc microparticles were found inside the pellets under higher magnification (Fig. 1f). As a result, pellets with talc addition were darker than those without talc (Fig. 1). The detailed morphological characteristics of pellets with different talc dosage were listed in Table 1. It is observed that pellet diameter decreased distinctly in the presence of talc, dropping from 297.63 ± 156.33 μm of control to 205.65 ± 71.56 μm of 10 g L−1 talc supplementary, with 30.90% decrease. However, pellet size decrease was not obvious with the further increase of talc concentration to above 5 g L−1. Pellet diameter in the absence of talc had a higher standard deviation, which indicated that pellet size were relatively heterogeneous. Nevertheless, pellet size became homogeneous in the presence of talc. Besides, the ratio of major axis/minor axis gradually increased with the increase of talc concentration, suggesting that pellets were becoming elongated. Similarly, compactness as an indicator of pellet luminousness increased when talc concentration was added, indicating that more talc microparticles were trapped inside the pellets.| Talc content (g L−1) | Mean diameter (μm) | Major axis/minor axis | Compactness |
|---|---|---|---|
| a For each talc concentration, at least 150 pellets from three replicates were analyzed. Data were mean values and standard deviations from each sample. | |||
| 0 | 297.63 ± 156.33 | 1.78 ± 0.40 | 0.31 ± 0.11 |
| 5 | 210.92 ± 75.35 | 1.81 ± 0.42 | 0.40 ± 0.17 |
| 10 | 205.65 ± 71.56 | 1.92 ± 0.54 | 0.42 ± 0.18 |
| 15 | 219.83 ± 76.10 | 1.96 ± 0.50 | 0.47 ± 0.18 |
| 20 | 214.90 ± 69.40 | 1.98 ± 0.44 | 0.56 ± 0.17 |
Grimm et al.19 had reported that spore aggregation is the key process for pellet formation. Addition of talc could prevent and disrupt spore aggregation by physical collision at the beginning of spore germination, and therefore decrease pellet size. When talc concentration was adequate freely dispersed mycelia and even short hyphal fragments could be obtained.6 Above all, the added talc microparticles should be comparable to the spores of filamentous microorganism in size. Kaup et al.20 demonstrated that microparticles in the size of about 500 μm show no effect on mycelia morphology of Caldariomyces fumago, while microparticles of ≤42 μm lead to the dispersion of mycelia up to single hyphae. Moreover, Driouch et al.6 also reported that the most pronounced effects on mycelia morphology are attributed to the smallest microparticles. The spore size of strain Streptomyces sp. M-Z18 is about 0.5–0.8 μm, which is about tenfold smaller than that of the filamentous fungi, e.g. Caldariomyces fumago. Therefore, we could deduce that microparticle in the size of ≤4.2 μm could lead to the fully dispersed mycelia of Streptomyces sp. M-Z18. The talc microparticles used in this study occupied the diameter of 2–12 μm (D50 ∼ 6 μm), while particle size of ≤4.2 μm only accounts for about 17.44% of the overall distribution (ESI Fig. S1†). Therefore, the effects of the used talc microparticles on mycelia morphology of Streptomyces sp. M-Z18 could be divided into two aspects: (1) a fraction of the talc microparticles with smaller size would effectively collide with the spore aggregation and decrease its scale; (2) the bigger microparticles could intertwine with the mycelia formed from spore germination and presented inside the pellets, which obviously created a loose interior structure with a better biomass filling in the pellet core.5 As a result, pellets with smaller size and darker inside were formed in the presence of talc, whereas, freely dispersed mycelia could not be formed even though the talc concentration was high enough (Fig. 1). In fact, freely dispersed mycelia even could not be formed in the cultivation of filamentous fungi (Aspergillus ficuum and Aspergillus terreus ATCC 20542) with talc addition, which might also attribute to the size distribution of the selected microparticles.21,22 Moreover, the physical collisions caused by the talc addition made the degree of spore aggregation more uniform, which eventually homogenized pellet size (Table 1). Besides, the pellet morphology observed was the combined effects of cell-particle collisions and shear stress exerted by the microparticles upon energy input by rotational shaking of the shake-flask.20 This could also explain why the peripheral mycelia decreased with the incremental talc addition.
3.2 Influence of talc microparticles on ε-PL production and cell growth in shake-flask fermentation
After considering the influence of talc microparticles on mycelia morphology, we further monitored the performances of ε-PL production and cell growth under different talc concentrations (Table 2). It is observed that both ε-PL production and cell growth were enhanced by talc addition. Biomass increased with talc concentration, and the maximum DCW of 7.05 ± 0.13 g L−1 was obtained at 20 g L−1 of talc addition. This finding was in perfect accordance with the cultivation of Aspergillus terreus ATCC 20542 in shake-flask fermentation with talc addition.22 ε-PL production was highest in the culture containing 10 g L−1 talc microparticles (2.51 ± 0.08 g L−1), which was increased by 50.30%, compared to the control (1.67 ± 0.11 g L−1). The further increase of talc concentration would hinder ε-PL production, but still make it higher than that of the control. Besides, the addition of talc microparticles could also facilitate glycerol consumption during the fermentation. There was about 83% glycerol (49.91 ± 1.78 g L−1) unconsumed after 72 h cultivation in the control, whereas, about 50% glycerol (29.98 ± 2.33 g L−1) was unconsumed when 20 g L−1 talc was supplemented. It is observed that small pellets with loose interior structure were obtained with talc addition (Fig. 1). As is known, small pellets occupied high specific surface area as well as the loose interior structure, which will facilitate the transfer of oxygen and nutrients from medium to mycelia.5 Consequently, glycerol consumption was accelerated in the presence of talc microparticles. Moreover, ε-PL production is a high energy consuming process,23 the adequate oxygen and nutrient supply will provide more ATP and precursors for the enhancing of ε-PL production and cell growth.3.3 Batch fermentations for ε-PL production in the absence and presence of talc microparticles
As shown above, talc addition at 10 g L−1 could adjust mycelia morphology for the optimized ε-PL production in shake-flask fermentation. Therefore, batch fermentations for ε-PL production with 10 g L−1 talc addition and without addition (control) were evaluated in a 5 L fermenter. Likewise, talc addition had distinctly changed mycelia morphology in the fermenter, resulting in small and elongated pellets (Fig. 2). Pellet size in both fermentations undergone the same process of first increase and then decrease, whereas, pellet size in the presence of talc microparticles was much smaller than that of the control. Pellet shape in the absence of talc stabilized throughout the fermentation, while pellet shape in the presence of talc became more and more elongated along with the fermentation period. | ||
| Fig. 2 Distribution of size and shape of pellets in batch fermentations for ε-PL production by Streptomyces sp. M-Z18 without (a–c) and with (d–f) addition of talc microparticles (6 μm, 10 g L−1). | ||
During the cultivation, pH spontaneously declined from initial 6.8 to the set value of 3.8, and then it was maintained stable by ammonia water (12.5%, w/v) until the end of cultivation. It is also observed that 10 g L−1 talc addition showed little impact on pH decline (Fig. 3a). Notably, talc addition had significantly stimulated the metabolic activity of Streptomyces sp. M-Z18, indicated by the accelerated cell growth, ε-PL production and glycerol consumption (Fig. 3b–d). As a result, fermentation time was shortened from 54.5 to 39 h. Moreover, cell growth, ε-PL production and glycerol consumption rates with talc addition reached 0.32, 0.16 and 1.68 g L−1 h−1, which were 23.08%, 23.08% and 43.59% higher than those of the control, respectively. Besides, the increased oxygen demand also confirmed the stimulating effect of talc addition (ESI Fig. S2†). To meet the demand for dissolve oxygen, agitation speed in the presence of 10 g L−1 talc microparticles increased much faster than that of the control. To further analyze the effects of talc addition on cell growth and ε-PL production, specific cell growth rate (μx) and specific ε-PL production rate (μp) were calculated based on the data in Fig. 3b and c. As shown in Fig. 3e and f, μx was slightly promoted in the presence of 10 g L−1 talc, moreover, the promotion for μp was much more pronounced. ε-PL production with 10 g L−1 talc addition attained the highest μp at about 21 h with 0.03 h−1, whereas, ε-PL production without talc addition attained the highest μp at about 26 h with 0.02 h−1. This result was in agreement with fructofuranosidase production by Aspergillus niger SKAn1015, in which the specific fructofuranosidase production rate with talc addition was almost fourfold higher than that of the control.6 Thereby, talc microparticles could also adjust pellet morphology for the promotion of ε-PL production and cell growth in fermenter.
3.4 Production of ε-PL with microparticle addition in fed-batch fermentation
Fed-batch fermentation is an essential course for sufficient ε-PL production. The potential of talc addition on cell growth and ε-PL production was now considered in fed-batch fermentation. Fig. 4 displayed the time profiles of fed-batch fermentations for ε-PL production by Streptomyces sp. M-Z18 with and without (control) addition of 10 g L−1 talc microparticles. It is observed that kinetic parameters of this microparticle-enhanced process differed dramatically from the control. Both glycerol and NH4+-N consumptions were promoted by talc addition. Glycerol feeding started at 36 h with talc addition, while it was delayed 12 h without talc. Similarly, NH4+-N feeding in the presence of talc started at 66 h, which was 24 h earlier than that of the control. The NH4+-N form is the most effective nitrogen source for ε-PL production, which could combine with oxaloacetate to generate precursor L-lysine.24 Most strikingly, ε-PL production and cell growth rates with talc microparticles were also accelerated during the feeding phase of fed-batch fermentation. As a result, ε-PL production and biomass reached 30.57 and 37.14 g L−1 at 168 h with supplemented talc microparticles, which were increased by 44.06% and 5.99%, respectively, compared with the control. In the presence of talc microparticles, small pellets with loose interior structure were obtained, whereas, pellets with large size and dense layer were formed without talc addition. Obviously, the supply of oxygen and nutrients to cells with talc addition was facilitated, compared to the control. Thus, mycelia in the small pellets occupied higher activity.5,6 Consequently, glycerol and NH4+-N consumptions, cell growth and ε-PL production were enhanced with talc supplementary. Besides, the sufficient supply of oxygen could also decrease the formation of organic acids (by-products). It has been reported that organic acids could be accumulated through TCA circle under oxygen limitation.25 Gao et al. reported that the amounts of malate are substantially decreased when microparticles are added in the production of lipid by Mortierella Isabellina.26 The same phenomenon was also found in the production of glucoamylase and fructofuranosidase by Aspergillus niger, in which the formation of oxalate was insignificant in the microparticle-enhanced cultivation.6 Unfortunately, the by-products of ε-PL production were still unclear by now. However, we still consider that organic acids or their polymers account for high shares of by-products, because of the sustained decline of pH in ε-PL production. Therefore, talc addition might also decrease the accumulation of organic acids in this study. These results demonstrated that talc addition could also be an efficient strategy for the enhancement of ε-PL production in fed-batch fermentation. | ||
| Fig. 4 ε-PL production by Streptomyces sp. M-Z18 in fed-batch fermentations without (a) and with (b) addition of talc microparticles (6 μm, 10 g L−1) in a 5 L fermenter. | ||
3.5 Fed-batch fermentation for ε-PL production with acidic pH shock in the presence of talc microparticles
In our previous work, acidic pH shock integrated with agro-industrial by-products utilization have been successfully employed for the overproduction of ε-PL in fed-batch fermentation. The whole process of fed-batch fermentation could be divided into three phases. Phase 1, pre-acid-shock adaption, pH was maintained at 5.0 until DCW was doubled for the induction of acid tolerance response (ATR), which would alleviate the damage that caused by the extremely low pH value of the followed pH-shock phase. Phase 2, acidic pH shock, pH declined from 4.0 to 3.0 and maintained for 12 h, which would regulate the metabolic activity of mycelia. Phase 3, recovery, pH was restored to 4.0 for the subsequent fermentation, which would provide optimal condition for ε-PL production. The proposed strategy was benefit for industrial application for the highest ε-PL production and low raw material cost.7 To this end, the influence of acidic pH shock on ε-PL production from agro-industrial by-products with 10 g L−1 talc addition was investigated (Fig. 5). All other process parameters remained unchanged. After 192 h of fed-batch fermentation, ε-PL production and biomass reached 62.36 and 78.13 g L−1, respectively, compared to 54.70 and 76.35 g L−1 of the control without talc.7 In conclusion, microparticle-based morphology engineering is a powerful and universal tool for enhancing ε-PL production. The presented bioprocess strategy would provide important information towards future industrial ε-PL production.4. Conclusions
These results indicated that talc microparticles could change the morphology of Streptomyces sp. M-Z18 and improve ε-PL production. In the presence of talc microparticles, small pellets with loose interior structure were obtained. As a result, oxygen and nutrition supply could be enhanced, which would promote the activity of mycelia in terms of glycerol and NH4+-N consumptions, cell growth and ε-PL production. Therefore, ε-PL production in shake-flask fermentation was increased from 1.67 ± 0.11 g L−1 of the control to 2.51 ± 0.08 g L−1 with 10 g L−1 talc microparticles. Moreover, ε-PL production in the presence of 10 g L−1 talc microparticles reached 30.57 g L−1 after 168 h of fed-batch fermentation in a 5 L fermenter, with 44.06% increase over the control. Additionally, when 10 g L−1 talc microparticles were supplemented to the fed-batch fermentation with acidic pH shock and agro-industrial by-products, the final ε-PL production was increased to 62.36 g L−1 at 192 h. In conclusion, the application of talc microparticles is an encouraging approach for ε-PL production.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31301556, 21376106), the Jiangsu Key Project of Scientific and Technical Supporting Program (BE2012616), the Cooperation Project of Jiangsu Province among Industries, Universities and Institutes (BY2013015-11), the Jiangsu Province ‘‘Collaborative Innovation Center for Advanced Industrial Fermentation’’ Industry Development Program, the Priority Academic Program Development of Jiangsu Higher Education Institutions and the 111 Project (111-2-06).References
- L. F. Dobson, C. C. O'Cleirigh and D. G. O'Shea, Appl. Microbiol. Biotechnol., 2008, 79, 859–866 CrossRef CAS PubMed.
- R. Walisko, R. Krull, J. Schrader and C. Wittmann, Biotechnol. Lett., 2012, 34, 1975–1982 CrossRef CAS PubMed.
- G. P. van Wezel, P. Krabben, B. A. Traag, B. J. Keijser, R. Kerste, E. Vijgenboom, J. J. Heijnen and B. Kraal, Appl. Environ. Microbiol., 2006, 72, 5283–5288 CrossRef CAS PubMed.
- K. P. Singh, P. P. Wangikar and S. Jadhav, J. Ind. Microbiol. Biotechnol., 2012, 39, 27–35 CrossRef CAS PubMed.
- H. Driouch, R. Hänsch, T. Wucherpfennig, R. Krull and C. Wittmann, Biotechnol. Bioeng., 2012, 109, 462–471 CrossRef CAS PubMed.
- H. Driouch, B. Sommer and C. Wittmann, Biotechnol. Bioeng., 2010, 105, 1058–1068 CAS.
- X. D. Ren, X. S. Chen, X. Zeng, L. Wang, L. Tang and Z. G. Mao, Bioprocess Biosyst. Eng., 2015, 38, 1113–1125 CrossRef CAS PubMed.
- S. Shima and H. Sakai, Agric. Biol. Chem., 1977, 41, 1807–1809 CrossRef CAS.
- S. Shima, H. Matsuoka, T. Iwamoto and H. Sakai, J. Antibiot., 1984, 37, 1449–1455 CrossRef CAS.
- I. L. Shih, M. H. Shen and Y. T. van, Bioresour. Technol., 2006, 97, 1148–1159 CrossRef CAS PubMed.
- P. Kahar, T. Iwata, J. Hiraki, E. Y. Park and M. Okabe, J. Biosci. Bioeng., 2001, 91, 190–194 CrossRef CAS.
- X. S. Chen, S. Li, L. J. Liao, X. D. Ren, F. Li, L. Tang, J. H. Zhang and Z. G. Mao, Bioprocess Biosyst. Eng., 2011, 34, 561–567 CrossRef CAS PubMed.
- Y. Zhang, X. Feng, H. Xu, Z. Yao and P. Ouyang, Bioresour. Technol., 2010, 101, 5523–5527 CrossRef CAS PubMed.
- S. Liu, Q. Wu, J. Zhang and S. Mo, Biotechnol. Lett., 2011, 33, 1581–1585 CrossRef CAS PubMed.
- X. Chen, L. Tang, S. Li, L. Liao, J. Zhang and Z. Mao, Bioresour. Technol., 2011, 102, 1727–1732 CrossRef CAS PubMed.
- X. D. Ren, X. S. Chen, L. Tang, Q. X. Sun, X. Zeng and Z. G. Mao, Ann. Microbiol., 2015, 65, 733–743 CrossRef CAS.
- R. Itzhaki, Anal. Biochem., 1972, 50, 569–574 CrossRef CAS.
- AOAC International (formerly the Association of Official Analytical Chemists), Official Methods of Analysis Arlington, AOAC International, VA., 1995.
- L. Grimm, S. Kelly, J. Hengstler, A. Göbel, R. Krull and D. Hempel, Biotechnol. Bioeng., 2004, 87, 213–218 CrossRef CAS PubMed.
- B. A. Kaup, K. Ehrich, M. Pescheck and J. Schrader, Biotechnol. Bioeng., 2008, 99, 491–498 CrossRef CAS PubMed.
- H. B. Coban, A. Demirci and I. Turhan, Bioprocess Biosyst. Eng., 2015, 38, 1075–1080 CrossRef CAS PubMed.
- J. Gonciarz and M. Bizukojc, Eng. Life Sci., 2014, 14, 190–200 CrossRef CAS PubMed.
- K. Yamanaka, N. Kito, Y. Imokawa, C. Maruyama, T. Utagawa and Y. Hamano, Appl. Environ. Microbiol., 2010, 76, 5669–5675 CrossRef CAS PubMed.
- M. Takehara and H. Hirohara, in Amino-Acid Homopolymers Occurring in Nature, Springer-Verlag, Berlin, Germany, 2010, pp. 1–22 Search PubMed.
- L. Karaffa and C. P. Kubicek, Appl. Microbiol. Biotechnol., 2003, 61, 189–196 CrossRef CAS PubMed.
- D. Gao, J. Zeng, X. Yu, T. Dong and S. Chen, Biotechnol. Bioeng., 2014, 111, 1758–1766 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Fig. S1 and S2. See DOI: 10.1039/c5ra14319e |
| This journal is © The Royal Society of Chemistry 2015 |