DOI:
10.1039/C5RA14299G
(Paper)
RSC Adv., 2015,
5, 84856-84864
Electrochemical degradation of perfluorooctanoic acid (PFOA) by Yb-doped Ti/SnO2–Sb/PbO2 anodes and determination of the optimal conditions†
Received
20th July 2015
, Accepted 28th September 2015
First published on 28th September 2015
Abstract
Model aqueous solutions of perfluorooctanoic acid (PFOA, 100 mg L−1) were electro-oxidized in a homemade container. The electrocatalytic behavior and anodic performance of Ti/SnO2–Sb/Yb–PbO2, Ti/SnO2–Sb–PbO2 and Ti/SnO2–Sb–Yb anodes in sodium electrolytes were compared. The SnO2–Sb/Yb–PbO2 anode demonstrated better electrocatalytic performance compared with the SnO2–Sb–PbO2 and SnO2–Sb–Yb electrodes in terms of both degradation and defluorination. Then a systematic experimental study was designed as follows to analyze the influencing factors: initial concentration of PFOA (10 mg L−1 to 200 mg L−1), current density (1 mA cm−2 to 40 mA cm−2), initial pH value (3 to 11) and electrode distance (5 mm to 20 mm). After a 150 min electrolysis, the optimum reaction conditions were obtained and the degradation and defluorination ratios reached 95.11 ± 3.9% and 75.7 ± 2.8%, respectively. Under the optimum conditions, the degradation of PFOA followed pseudo-first-order kinetics (0.0193 min−1) and the degradation half-life was 35.9 min. The produced F− was measured using a fluoride ion selective electrode, whereas the intermediate PFCAs with short-chain lengths were measured using HPLC-MS. A detailed degradation pathway was proposed in this study by analyzing the intermediates and the recovery of fluoride.
1. Introduction
Perfluorooctanoic acid compounds are considered important surfactants and emulsifiers because of their hydrophobic and oleophobic characteristics. Since the 1960s, the electrochemical fluorination method has been applied in the production of perfluorinated compounds, perfluorooctanoic acid, and many other kinds of perfluorinated carboxylic acids (PFCAs) and salts that contain sulfonyl perfluorinated organic compounds. Over recent decades a large number of these products have been developed for both industrial and domestic uses.1,2 The extensive use of these products and the strong bond energy of the C–F bond (116 kcal mol−1)3 have driven the accumulation of these compounds in various environmental niches worldwide.4–6 In fact, these compounds have also been observed in many animal7 and human tissues8,9 because of the amplification of the food chain. Some reports suggested that this class of compounds are toxic to experimental animals and humans.10,11 Therefore, in the fourth session of the Stockholm Conference in 2009, perfluorinated compounds including perfluorooctane sulfonic acid, perfluorooctane sulfonate and perfluorooctanesulfonyl fluoride were included in the new list of persistent organic pollutants thereby establishing the harmfulness of such substances.
Several methods for degrading perfluorinated acids under specific situations have been investigated. Among these methods, sonochemical degradation12 and thermolysis13 can achieve high decomposition ratios for PFOA, but these techniques require strict reaction conditions and high energy consumption. Although the persulfate radical (S2O82−) oxidation14 and H3PW12O40 (ref. 15) methods can degrade PFOA under mild reaction conditions, the defluorination process is slow and inefficient. Membrane separation,16,17 adsorption18,19 and ion exchange20,21 have also been investigated, but these merely transfer the contaminants to a second phase. The effect of disposal of the generated waste disposal on the environment and the consumption of resources must also be managed.
Unlike the above methods, electrochemical oxidation can overcome the limited oxidizing abilities of conventional advanced oxidation processes because of its many advantages, such as high oxidation efficiency, fast reaction rate, easy operation, amenable to automation, and environmental compatibility. Previous studies have demonstrated the effectiveness of boron-doped diamond, SnO2 and PbO2 in the degradation and mineralization of perfluorocarboxylic and perfluorosulfonic acids in a model solution.22 Some reports have also reported the strong ability of the lead dioxide electrode in producing hydroxyl radical.23–26 Other researchers have incorporated materials, such as Nb5+,27 Bi4+,28 Ce4+,29 Mn4+,30 and carbon aerogel31 into the PbO2 coating to enhance its catalytic activity for wastewater treatment.32
In the previous report,32,33 cerium was doped in the preparation of electrodes for degrading PFOA. Ytterbium and cesium, which both belong to lanthanide, share the similar chemical properties. The configuration of ytterbium is [Xe]4f146s2, which is the most stable element among the rare earth (RE) element because of the saturation state. At the same time, the resistivity of ytterbium is 28 μΩ cm−1, which is the smallest among the rare earth. The structural superiority above makes Yb3+ being studied extensively for many applications, such as the preparation of lithium ion batteries.34 The photoluminescence properties of Yb-doped Si oxide35 have been investigated, but the performance of Yb-doped electrode in degrading PFOA by electrochemical method has been rarely examined.
In this study, we attempt to decompose PFOA using a novel Yb(III)-doped PbO2 anode that is selected from three types of anodes (e.g., Ti/SnO2–Sb/Yb–PbO2, Ti/SnO2–Sb–PbO2 and Ti/SnO2–Sb–Yb) and propose a decomposition pathway. We prepared and doped the electrode, investigated the effects of different factors on the electrochemical decomposition and defluorination of PFOA and studied the decomposition kinetics and defluorination pathways of PFOA by measuring the degradation rate and identifying the intermediate products. The Yb-doped anode can be an effective technology for dealing with PFOA pollution in the future.
2. Material and methods
2.1. Chemical reagents and instruments
SnCl4·5H2O (99%, Hushi, Shanghai), SbCl3 (98%, Kemiou, Tianjin), HCl (36%, Tieta, Shandong), Pb(NO3)2 (99%, Damao, Tianjin), iso-propanol (99.7%, Fuyu, Tianjin), HNO3 (68%, Kangde, Laiyang), NaF (99%, Damao, Tianjin), Yb(NO3)3·6H2O (99%, Bailingwei, Beijing), oxalic acid (99%, Xilong, Guangdong), NaOH (96%, Yongda), Na2SO4 (99%, Tianda, Tianjin) and Ti sheet (99%, Taiye, Baoji) were used to prepare the electrodes and for the electrolysis procedures. Perfluorooctanoic acid (PFOA, 95%) was purchased from Wongjiang (China). Perfluoropropanoic acid (PFPrA, 97%) was supplied by Aladdin (China). Perfluoroheptanoic acid (PFHpA, 98%), perfluorohexanoic acid (PFHxA, 98%), perfluoropentanoic acid (PFPeA, 97%), perfluorobutanoic acid (PFBA, 98%) and trifluoroacetic acid (TFA, 99%) were obtained from Bailingwei (China). Na3C6H5O7·2H2O (98%, Beichen, Tianjin) and NaNO3 (98%, Aibi, Shanghai) were used when measuring the concentration of fluorine ion. The instruments involved during the experiment as follows: ultrasonic instrument (KQ-250B, Kunshanyiqi), drying cabinet (101A-2, Shuangwujin), muffle furnace (XS1-25-1200, Zhongda), pH meter (PXS-215, Lieci), magnetic stirrer (79-1, Guohua), DC power supply (DYY-6B, Liuyi). All chemicals were used without further purification and deionized water was used in all of the experiments.
2.2. Electrode fabrication
The titanium (Ti) sheet was cut to a rectangular shape (50 mm × 20 mm, with a thickness of 0.4 mm). After polishing with two grades of sandpaper (280-grid and 600-grid), the sheets were placed in the ultrasonic instrument for 10 min to remove the particles on its surface. The sheet was then soaked in a NaOH solution (5%, m/m) at 95 °C for 1 h to remove grease. The Ti sheet was washed with distilled water and etched in boiling oxalic acid solution (10%, m/m) for about 2 h until gray pits were observed on its surface. The middle layers of the Ti/SnO2–Sb/Yb–PbO2 and Ti/SnO2–Sb–PbO2 anodes are the same, while that of the Ti/SnO2–Sb–Yb anode is different. The coating solution was prepared as follows using the sol–gel technique: SnCl4·5H2O and SbCl3 were dissolved in 50 mL iso-propanol at a Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb atomic ratio of 95
Sb atomic ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Next, 15 mL of concentrated HCl solution was added, and the resulting solution was diluted to 100 mL using iso-propanol. This solution was marked as solution A. For the Ti/SnO2–Sb–Yb anode, we added trace ytterbium nitrate to the iso-propanol solution (which contained dissolved Sn and Sb) at a Sn
5. Next, 15 mL of concentrated HCl solution was added, and the resulting solution was diluted to 100 mL using iso-propanol. This solution was marked as solution A. For the Ti/SnO2–Sb–Yb anode, we added trace ytterbium nitrate to the iso-propanol solution (which contained dissolved Sn and Sb) at a Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb atomic ratio of 95
Yb atomic ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. This solution was marked as solution B and was used to make the Ti/SnO2–Sb–Yb electrode only. We prepared three identical Ti sheets, of which two (marked as sheets 1 and 2, respectively) were dipped in solution A, while the other sheet (sheet 3) was dipped in solution B for 5 min. The sheets were then dried at 130 °C for 10 min, and the organics were thermally decomposed at 490 °C for 5 min. This dipping–annealing cycle was repeated 12 times. In the final stage, the coating was annealed for 1 h at 490 °C. Sheet 3 was the Ti/SnO2–Sb–Yb electrode. Electro-deposition was employed for the preparation of Ti/SnO2–Sb/Yb–PbO2 and Ti/SnO2–Sb–PbO2. Sheet 1 was placed into an acidic (pH 1, adjusted with HNO3) electro-deposition solution, which contained 200 g L−1 Pb(NO3)2, 2 g L−1 NaF, and 2.36 g L−1 Yb(NO3)3·6H2O at a constant anode current density of 20 mA cm−2 for 90 min under room temperature (25 °C). The anode was used as the Ti/SnO2–Sb/Yb–PbO2 electrode. Sheet 2 was treated similarly as sheet 1 except without the addition of Yb(NO3)3. The preparation of Ti/SnO2–Sb–PbO2 was thus completed.
0.5. This solution was marked as solution B and was used to make the Ti/SnO2–Sb–Yb electrode only. We prepared three identical Ti sheets, of which two (marked as sheets 1 and 2, respectively) were dipped in solution A, while the other sheet (sheet 3) was dipped in solution B for 5 min. The sheets were then dried at 130 °C for 10 min, and the organics were thermally decomposed at 490 °C for 5 min. This dipping–annealing cycle was repeated 12 times. In the final stage, the coating was annealed for 1 h at 490 °C. Sheet 3 was the Ti/SnO2–Sb–Yb electrode. Electro-deposition was employed for the preparation of Ti/SnO2–Sb/Yb–PbO2 and Ti/SnO2–Sb–PbO2. Sheet 1 was placed into an acidic (pH 1, adjusted with HNO3) electro-deposition solution, which contained 200 g L−1 Pb(NO3)2, 2 g L−1 NaF, and 2.36 g L−1 Yb(NO3)3·6H2O at a constant anode current density of 20 mA cm−2 for 90 min under room temperature (25 °C). The anode was used as the Ti/SnO2–Sb/Yb–PbO2 electrode. Sheet 2 was treated similarly as sheet 1 except without the addition of Yb(NO3)3. The preparation of Ti/SnO2–Sb–PbO2 was thus completed.
2.3. Electrochemical experiments
The 200 mL electrochemical reaction container was made of organic glass (polymethyl methacrylate). During the experiments, 200 mL PFOA (100 mg L−1) was electrolyzed using 0.1 M Na2SO4 as a supporting electrolyte. The prepared Ti/SnO2–Sb/Yb–PbO2, Ti/SnO2–Sb–PbO2 and Ti/SnO2–Sb–Yb electrodes were used as the anodes, and a same size Ti sheet was used as the cathode. The effective electrolytic area was 10 cm2, and the stirring rate was 500 rpm. The distance between the two electrodes and the electrolysis current density can be changed. The reaction solution was sampled for analysis every 15 min or 30 min during the experiments. All experiments were performed triplicate and performed at room temperature.
2.4. Instrumental analysis
The PFCAs concentration were measured by using a high-performance liquid chromatography-mass spectrometry (HPLC-MS, Dionex U3000, USA), which was equipped with a Thermo C18 column (100 mm × 2.1 mm, 3 μm). A mixture of methanol (80%, volume percent) and distilled water (20%, containing 0.1% methanoic acid) was used as the mobile phase. The sample injection volume was 10 μL at a flow rate of 0.2 mL min−1. Room temperature was regarded as the column temperature (25 °C). Electrospray negative ionization mass spectrometry (Thermo LCQ Fleet, USA) was used to identify the intermediate products of PFOA. The capillary temperature was 320 °C. The full scan range of the mass spectra ranged from 100 m/z to 600 m/z. The gas flow rates of sheath, aux, and sweep were 30, 10 and 0 units, respectively, and the spray voltage was −4 kV. Given that the sodium sulfate electrolyte could harm the mass spectrometer, solid-phase extraction was performed to remove the salt from all samples before measurement. The analyte-specific mass spectra and standard curves of the PFCAs are shown in Fig. S1 and S2 (a to g) in the ESI section.†
The concentration of F− was measured using a fluoride ion selective electrode. Given that the acidity of the electrolyzed solution (pH < 4) affects the determination of fluoride ion concentration, the total ion strength adjustment buffer (TISAB) should be added to the test solution. TISAB was prepared as follows: 58.8 g sodium citrate and 85 g sodium nitrate were dissolved in a beaker, then the pH was adjusted between 5 and 6 with HCl, and the solution was diluted to 1000 mL using distilled water. The concentration of fluoride (CF−) and the defluorination ratio (R) were calculated as follows:
| |
 | (1) |
| |
 | (2) |
where
E0 (mV) represents the steady electromotive force,
E (mV) is the determined electromotive force,
CF− is the concentration of fluoride ion (mg L
−1), and
C0 is the initial concentration of PFOA in mg L
−1. The stoichiometric factor of 15 indicates to the number of fluorine atoms in a PFOA molecule. Under the condition that the total ion strength in the solution is constant and sufficient, the electromotive force (
E) has a linear relationship with log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CF−
CF− and

represents the slope of this line. The concentration of F
− can be obtained from the standard curve of F
− (Fig. S3
†).
| |
 | (3) |
The average current efficiency (ACE) is calculated by the formula as above. Where COD0 and CODt are the chemical oxygen demands (mg L−1) at the initial and the final moments, respectively. The difference between COD0 and CODt is COD removed. F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 C mol−1), V is the volume of electrolyte (L), 8 is the equivalent mass of oxygen (g mol−1), I is the current (A), and t means the electrolysis time (s).
487 C mol−1), V is the volume of electrolyte (L), 8 is the equivalent mass of oxygen (g mol−1), I is the current (A), and t means the electrolysis time (s).
3. Results
3.1. Selection of the anode material
As shown in Fig. 1, under the standard reaction conditions, the degradation ratios of PFOA were 95.11 ± 3.9%, 83.94 ± 1.6, and 80.14 ± 3.8% for the Ti/SnO2–Sb/Yb–PbO2, Ti/SnO2–Sb–PbO2 and Ti/SnO2–Sb–Yb anodes, respectively. Kinetic behavior was observed and modeled as a probable pseudo-first-order reaction. The electrochemical degradation rate constants (k, k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/C) of the three anodes were 0.0193, 0.012 and 0.011 min−1, respectively, and their defluorination ratios of PFOA were 76.7 ± 2.8%, 61.4 ± 1.1% and 52.8 ± 2.8%, respectively, as shown in Fig. 1. According to the rate constants, the degradation of the optimal reaction conditions (Table 1). A 200 mL PFOA solution was used in the electrochemical experiments to examine the effects of current density (1 mA cm−2 to 40 mA cm−2), initial concentration (10 mg L−1 to 200 mg L−1), electrode distance (5 mm to 20 mm), and initial pH (3 to 7).
C0/C) of the three anodes were 0.0193, 0.012 and 0.011 min−1, respectively, and their defluorination ratios of PFOA were 76.7 ± 2.8%, 61.4 ± 1.1% and 52.8 ± 2.8%, respectively, as shown in Fig. 1. According to the rate constants, the degradation of the optimal reaction conditions (Table 1). A 200 mL PFOA solution was used in the electrochemical experiments to examine the effects of current density (1 mA cm−2 to 40 mA cm−2), initial concentration (10 mg L−1 to 200 mg L−1), electrode distance (5 mm to 20 mm), and initial pH (3 to 7).
 |
| | Fig. 1 Electrochemical degradation and defluorination ratio of PFOA at the reaction conditions including applied current density (20 mA cm−2), initial concentration (100 mg L−1), electrode distance (5 mm), initial pH (5) and with a 0.1 M sodium sulfate supporting electrolyte solution during 150 min, T = 25 °C. | |
Table 1 The efficiency and kinetics for PFOA degradation by Ti/SnO2–Sb/Yb–PbO2 electrode (electrolysis time: 150 min)a
| Parameters |
|
ηb |
kc |
t1/2d |
R2e |
| Other operating conditions are same as Fig. 3–6. Degradation ratio of PFOA (%). Pseudo-first-order rate constant of electrochemical degradation (min−1). The time of half-left (min). Goodness of fit. |
| Current density (mA cm−2) |
1 |
58.50 ± 3.1% |
0.0061 |
113.63 |
0.991 |
| 5 |
78.10 ± 2.7% |
0.0094 |
113.63 |
0.984 |
| 10 |
86.33 ± 3.6% |
0.0135 |
51.34 |
0.995 |
| 20 |
95.11 ± 3.9% |
0.0193 |
35.91 |
0.995 |
| 30 |
96.68 ± 2.1% |
0.0234 |
29.62 |
0.997 |
| 40 |
99.30 ± 0.2% |
0.0306 |
22.65 |
0.987 |
| Initial PFOA concentration (mg L−1) |
10 |
90.10 ± 0.1% |
0.0162 |
42.79 |
0.999 |
| 50 |
93.57 ± 0.7% |
0.0177 |
39.16 |
0.994 |
| 100 |
95.11 ± 3.9% |
0.0193 |
35.91 |
0.995 |
| 200 |
83.86 ± 2.8% |
0.0127 |
54.58 |
0.991 |
| Initial pH value |
3 |
87.65 ± 1.4% |
0.0139 |
49.87 |
0.994 |
| 5 |
95.11 ± 3.9% |
0.0193 |
35.91 |
0.995 |
| 7 |
87.13 ± 0.9% |
0.0130 |
53.32 |
0.990 |
| 9 |
78.34 ± 1.4% |
0.0103 |
67.30 |
0.994 |
| 11 |
62.71 ± 2.2% |
0.0067 |
103.45 |
0.988 |
| Gap distance (mm) |
5 |
95.11 ± 3.9% |
0.0193 |
35.91 |
0.995 |
| 10 |
89.05 ± 1.1% |
0.0151 |
45.90 |
0.998 |
| 15 |
85.23 ± 1.4% |
0.0122 |
56.82 |
0.986 |
| 20 |
67.92 ± 3.8% |
0.0079 |
87.74 |
0.985 |
3.2. Initial PFOA concentration
The effects of PFOA concentration are described in this section. Table 1 shows four samples with initial PFOA concentrations of 10, 50, 100 and 200 mg L−1. The concentrations of PFOA for the three anodes were reduced by 90.90 ± 0.1%, 93.75 ± 0.7%, 95.11 ± 3.9% and 83.86 ± 2.8%, respectively (Fig. 2(a)), indicating the excellent performance of the Ti/SnO2–Sb/Yb–PbO2 electrode in degrading PFOA for low-concentration solutions (<100 mg L−1).
 |
| | Fig. 2 (a) Effect of the initial PFOA concentration and (b) fitted pseudo-first-order kinetic curve (initial pH 5, plate distance 5 mm, current density 20 mA cm−2, 0.1 M Na2SO4, T = 25 °C). | |
Fig. 2(b), shows that with an increasing initial concentration of PFOA, the pseudo-first-order kinetic constant (k) is reduced at first, eventually increases; correspondingly, the electrochemical reaction rates increased and then decreased. This phenomenon might be explained as follows. When the concentration of initial PFOA is low (<100 mg L−1), the PFOA around the anode is degraded but cannot be replenished quickly. Given the turbulence in the electrolytic cell, the PFOA located far from the anode is degraded slowly, thereby affecting the degradation rate of PFOA. In contrast, higher concentrations of shorter-chain intermediates were produced under high concentrations of PFOA. The reaction of the intermediates would consume HO· and gather on the surface of the electrode to transfer electrons, which would restrain the degradation reaction of PFOA.
3.3. Effect of current density
By influencing the electron transfer capability and the hydroxyl radical production in electrolytic systems, the current density affects the degradation and defluorination rates of PFOA.30 Fig. 3(a) shows the different current densities (1, 5, 10, 20, 30, and 40 mA cm−2) used to investigate the degradation of 100 mg L−1 PFOA. The degradation rate is very low at a low current (1 mA cm−2), where less than 60% of the PFOA can be degraded after 150 minutes of electrolysis. Compared with this result, the degradation rate gradually increased with the increasing of applied current density. When the current density increased from 20 mA cm−2 to 40 mA cm−2, the rate of degradation remained above 95% and the k value increased greatly as well, to 0.0193 min−1, 0.0234 min−1 and 0.0306 min−1 respectively.
 |
| | Fig. 3 (a) PFOA concentration as a function of electrolysis time at current density 1, 5, 10, 20, 30, 40 mA cm−2. Initial pH 5, plate distance 5 mm, 0.1 M Na2SO4, T = 25 °C. (b) COD removed and average current efficiency (ACE) as a function of current density. | |
As we can see from Fig. 3(b), when the X coordinate is 20 mA cm−2, the Yb-doped electrode has the highest average current efficiency (ACE). The degradation of COD is low (<250 mg L−1) under a low current intensity (1, 5, 10 mA cm−2). However, in spite of the high removal, a low ACE was obtained as well when the current intensity is higher than 20 mA cm−2 because of the high power consumption. This experiment also proved that the 20 mA cm−2 is the best degradation current density in this electro-chemical system. This finding may be attributed to two reasons. First, the mass transfer rate of PFOA molecules toward the anode is limited and the degradation is inadequate. Second, the oxidation reaction at the anode is gradually enhanced by the increasing applied current density. As a result, the current oversupply leads to a lower average current efficiency (ACE).36,37
3.4. Effect of initial pH value
The electrochemical degradation of PFOA was evaluated for five integral initial pH values ranging from 3 to 11. Fig. 4 shows the degradation rates and the fitted pseudo-zero-order kinetic curves after 150 minutes at different initial pH values. When the pH value was 5, the best degradation rate of 95.11 ± 3.9% was achieved, which was 1.1 times higher than that in pH 3 and pH 7, 1.2 times higher than that in pH 9, and 1.5 times higher than that in pH 11. The PFOA solutions with a lower initial pH value had a higher oxidation rate than those with a higher initial pH value, thus indicating that the oxidation process was more favorable in acidic solutions. This conclusion also mentioned in previous literatures.38,39 However, low pH value will increase the concentration of H+ and hamper the positive direction to produce HO·. Therefore, the trend in Fig. 4 was present. Thus, pH 5 was the most beneficial pH value for the degradation of PFOA.
 |
| | Fig. 4 Effect of initial pH value. Initial concentration 100 mg L−1, current density 20 mA cm−2, plate distance 5 mm, 0.1 M Na2SO4, T = 25 °C. | |
3.5. Effect of gap distance between two plates
At the same current density, the effect of different electrode gap distances on degradation of PFOA is equivalent to that of electrode voltage. The degradation ratios of PFOA were 95.11 ± 3.9%, 89.05 ± 1.1%, 85.23 ± 1.4%, and 67.92 ± 3.8% for the plate distances of 5, 10, 15, and 20 mm, respectively (Fig. 5). As shown in Table 1, the highest k value (0.0193 min−1) was obtained at 5 mm, the k values of 0.0151 and 0.0122 min−1 were obtained at 10 and 15 mm, respectively; and the lowest k value (0.0193 min−1) was obtained at 20 mm. The t1/2 value ranged between 35.9 min and 87.7 min. These data indicate that within a certain distance, a shorter electrode space will lead to a higher degradation efficiency. However, the voltages between two electrodes were 2.4, 2.9, 3.2 and 3.8 V for the distances of 5, 10, 15, and 20 mm, respectively. The longer electrode distance, the more electrolysis time was required due to the longer diffusion distance. Unlike degradation, the defluorination of PFOA required higher potential to activate electron transfer. Energy-band theory argues that the electron transfer reaction can be activated33 when the energy level of an unoccupied electron given by the anode is lower than that of the highest occupied molecular orbital of PFOA.
 |
| | Fig. 5 PFOA concentration change as a function of electrolysis time at gap distance of 5, 10, 15, 20 mm. Initial pH 5, current density 20 mA cm−2, initial PFOA concentration 100 mg L−1, 0.1 M Na2SO4, T = 25 °C. | |
3.6. The production of intermediates
To investigate the production of intermediates during the electrochemical process, HPLC-MS was used to measure the concentration of PFCAs (C2 to C7). As shown in Fig. 6(a), six different shorter-chain perfluoroalkyl groups (e.g., TFA, PFPA, PFBA, PFPeA, PFHxA, and PFHpA) were identified and quantified by using the mass spectra, which were produced during PFOA degradation as the intermediates. Fig. 6(a) also shows the formation of intermediates over the electrolysis time. As we can see from the diagram, during 150 min of degradation, the concentrations of PFHpA (C7) and PFPeA (C6) reached their maximum values at 30 min and 60 min, respectively, which decreased afterward. The concentrations of PFPeA (C5) and PFBA (C4) increased slightly since the beginning of electrochemical reaction but began to decrease after reaching their peaks at 90 and 120 min, respectively. The concentrations of PFPA (C3) and TFA (C2) slowly increased throughout the electrolysis time. The observed degradation process is similar to that seen by other researchers.30,40 In summary, the appearance of the maximum concentrations of the intermediates follows the order of the number of carbon atoms and PFCAs, with longer carbon chains showing higher concentrations. These phenomena indicate that the intermediates with longer chains are formed at the beginning of electrochemical reaction and are further decomposed into shorter chain intermediates as the reaction continues.33 The incomplete degradation also indicates that some PFOA degradation products have not been fully decomposed.| |
 | (4) |
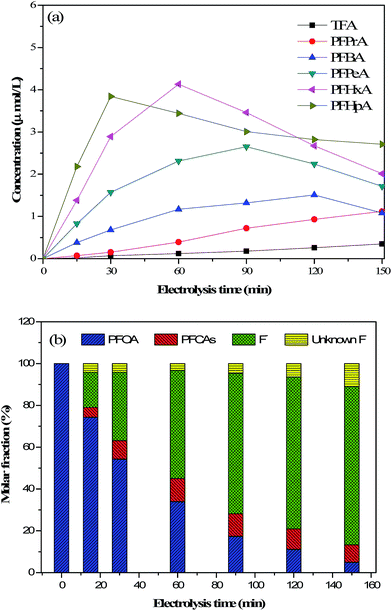 |
| | Fig. 6 (a) Concentration of intermediates (C2–C7) formed during electrochemical decomposition of PFOA and (b) fluorine element mass balance with the progress of reaction. The operation conditions are given in the caption of Fig. 1. | |
The mass balance of fluorine after 150 min electrolysis was investigated to analyze the electro-chemical degradation pathway of PFOA. Using eqn (3), the mass balance values of fluorine (i.e., {Fin intermediate PFCAs,undegraded− + Fin PFOA,undegraded− + Fin solution−}/Fin PFOA initial−), Fin intermediate PFCA,undegraded− were calculated to be 95.8%, 95.8%, 96.8%, 95.3%, 93.6%, and 89.0% at different times, as shown in Fig. 6(b). The recovery rates of fluoride were less than 100% (i.e., Funknown−), indicating that other intermediate products were quantified or detected in the solution. For example, volatile 1-C fluorocarbons may accumulate along with an increasing of electrolysis time.41 However, the high recovery rates of fluoride allow us to describe the main reaction mechanism of the PFOA degradation in an aqueous solution.
3.7. Degradation pathway of PFOA
Based on the results presented above and in the previous literature, a possible pathway by electrochemical oxidation could be expressed as follows: PFOA was ionized when dissolved in the solution (eqn (5)).42,43 Using the electrochemical device, PFOA underwent direct electron transfer from the carboxyl group on the anode to form perfluorooctanoic acid carboxyl radical (C7F15COO·) (eqn (6)). This radical was then decarboxylated to form the perfluoroheptyl radical (C7F15·), where the method of bond cleavage was similar to the Kolbe decarboxylation mechanism in eqn (7).44–46| | |
C7F15COOH + H2O → C7F15COO− + H3O+
| (5) |
| | |
C7F15COO− → C7F15COO· + e
| (6) |
| | |
C7F15COO· → C7F15· + CO2
| (7) |
After the degradation process, C7F15· followed two pathways. As shown in eqn (8), the perfluoroheptyl radical was oxidized by radical species to form C7F15OH,47,48 which could be largely produced by the typical active electrode.23 Given that C7F15OH was a terminally unstable alcohol,49 this radical underwent intramolecular rearrangement to form C6F13COF and eliminate HF (eqn (9)).15,50 The C6F13OF was then hydrolyzed to form shorter-chain PFCAs by removing of CF2 units15 and releasing fluoride ions into the aqueous solution (eqn (10)).
| | |
C7F15· + HO· → C7F15OH
| (8) |
| | |
C7F15OH → C6F13COF + H+ + F−
| (9) |
| | |
C6F13COF + H2O → C6F13COO− + 2H+ + F−
| (10) |
In the previous literature, another reaction pathway was proposed after the Kolbe decarboxylation. In eqn (11), the C7F15· reacted with the oxygen produced via water electrolysis.46 The existence of this pathway was proven by using oxygen isotope tracers.40 The produced C7F15COO· was then combined with another perfluorinated carboxylic acid radical (RFCOO·) to form C7F15CO· and RFCO· (eqn (12)).29 According to eqn (13), the C7F15CO· was decomposed to produce C7F13· and COF2.46 The obtained carbonyl fluoride was hydrolyzed to produce carbon dioxide and HF (eqn (14)). In the degradation process, some volatile fluorinated organic contaminants, such as CHF3, CF4 and C2F6, may be produced during the electrolysis of the PFCs.13,51
| | |
C7F15· + O2 → C7F15COO·
| (11) |
| | |
C7F15COO· + RFCOO· → C7F15CO· + RFCO· + O2
| (12) |
| | |
C7F15CO· → C7F13· + COF2
| (13) |
| | |
COF2 + H2O → CO2 + 2HF
| (14) |
In the two pathways above (eqn (5)–(10), and (5)–(7), (11)–(14)), PFOA was degraded to shorter-chain PFCAs by repeating the CF2− unzipping processes in a step-wise manner as time progressed, and PFOA was then entirely mineralized to CO2 and F−.
4. Conclusion
Typical anodes, such as Ti/SnO2–Sb–PbO2 and Ti/SnO2–Sb–Yb, can be used in the electrochemical degradation of PFOA, whereas the addition of the rare earth ytterbium (Ti/SnO2–Sb/Yb–PbO2 anode) can increase the decomposition of PFOA from 83.94 ± 1.6 and 80.14 ± 3.8% to 95.11 ± 3.9%. The reaction follows the pseudo-first-order kinetics, and the k values of PFOA follow the order of Ti/SnO2–Sb/Yb–PbO2 (0.0193 min−1) > Ti/SnO2–Sb–PbO2 (0.012 min−1) > Ti/SnO2–Sb–Yb (0.011 min−1). The degradation of PFOA showed a positive correlation with the increase of current density (1 mA cm−2 to 40 mA cm−2), reduction of initial concentration (10 mg L−1 to 200 mg L−1) and shorter electrode distance (5 mm to 20 mm). The degradation rate reached to the peak at pH 5, which was higher than the other tested pH values (3 to 11). The intermediate products of PFCAs (C2 to C7) and fluoride ion were detected, and a possible pathway for the PFOA electrochemical degradation was proposed by analyzing the intermediate products. Compared with other metal-doped electrodes that have been recently used (between 2011 and 2015) in the decomposition of PFOA, the linear fit of the kinetic plot was k = 0.0193 min−1, which was higher than that of the Mn-doped electrode (0.004 min−1),30 but lower than that of the Ce-doped (0.037 min−1) electrode.29 In addition, the degradation of the Yb-doped electrode was 95.1%, which was higher than the reduction of Bi-doped electrode (93.3%).28 These results indicate that electro-chemical oxidation with Yb-doped electrode is an efficient method for decomposing PFOA.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
The authors are grateful to the anonymous reviewers for their reading of the manuscript, and for their suggestions and critical comments.
References
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates, Environ. Sci. Technol., 2006, 40(1), 32–44 CrossRef CAS.
- T. Shao, P. Zhang, L. Jin and Z. Li, Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide, Appl. Catal., B, 2013, 142, 654–661 CrossRef PubMed.
- K.-J. Hansen, H. Johnson, J. Eldridge, J. Butenhoff and L. Dick, Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River, Environ. Sci. Technol., 2002, 36(8), 1681–1685 CrossRef CAS.
- M. So, S. Taniyasu, N. Yamashita, J. Giesy, J. Zheng, Z. Fang, S. Im and P. K. Lam, Perfluorinated compounds in coastal waters of Hong Kong, South China, and Korea, Environ. Sci. Technol., 2004, 38(15), 4056–4063 CrossRef CAS.
- K. Kannan, J. Koistinen, K. Beckmen, T. Evans, J. F. Gorzelany, K. J. Hansen, P. D. Jones, E. Helle, M. Nyman and J. P. Giesy, Accumulation of perfluorooctane sulfonate in marine mammals, Environ. Sci. Technol., 2001, 35(8), 1593–1598 CrossRef CAS.
- K. Kannan, J. Newsted, R. S. Halbrook and J. P. Giesy, Perfluorooctanesulfonate and related fluorinated hydrocarbons in mink and river otters from the United States, Environ. Sci. Technol., 2002, 36(12), 2566–2571 CrossRef CAS.
- J. Dai, M. Li, Y. Jin, N. Saito, M. Xu and F. Wei, Perfluorooctanesulfonate and perfluorooctanoate in red panda and giant panda from China, Environ. Sci. Technol., 2006, 40(18), 5647–5652 CrossRef CAS.
- J. M. Conder, R. A. Hoke, W. d. Wolf, M. H. Russell and R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds, Environ. Sci. Technol., 2008, 42(4), 995–1003 CrossRef CAS.
- M. K. So, N. Yamashita, S. Taniyasu, Q. Jiang, J. P. Giesy, K. Chen and P. K. S. Lam, Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China, Environ. Sci. Technol., 2006, 40(9), 2924–2929 CrossRef CAS.
- N. Johansson, A. Fredriksson and P. Eriksson, Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice, Neurotoxicology, 2008, 29(1), 160–169 CrossRef CAS PubMed.
- N. Johansson, P. Eriksson and H. Viberg, Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain, Toxicol. Sci., 2009, 108(2), 412–418 CrossRef CAS PubMed.
- H. Moriwaki, Y. Takagi, M. Tanaka, K. Tsuruho, K. Okitsu and Y. Maeda, Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid, Environ. Sci. Technol., 2005, 39(9), 3388–3392 CrossRef CAS.
- P. J. Krusic, A. A. Marchione and D. C. Roe, Gas-phase NMR studies of the thermolysis of perfluorooctanoic acid, J. Fluorine Chem., 2005, 126(11), 1510–1516 CrossRef CAS PubMed.
- H. Hori, Y. Nagaoka, M. Murayama and S. Kutsuna, Efficient decomposition of perfluorocarboxylic acids and alternative fluorochemical surfactants in hot water, Environ. Sci. Technol., 2008, 42(19), 7438–7443 CrossRef CAS.
- H. Hori, E. Hayakawa, H. Einaga, S. Kutsuna, K. Koike, T. Ibusuki, H. Kiatagawa and R. Arakawa, Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches, Environ. Sci. Technol., 2004, 38(22), 6118–6124 CrossRef CAS.
- J. Thompson, G. Eaglesham, J. Reungoat, Y. Poussade, M. Bartkow, M. Lawrence and J. F. Mueller, Removal of PFOS, PFOA and other perfluoroalkyl acids at water reclamation plants in South East Queensland Australia, Chemosphere, 2011, 82(1), 9–17 CrossRef CAS PubMed.
- Y.-T. Tsai, A. Yu-Chen Lin, Y.-H. Weng and K.-C. Li, Treatment of perfluorinated chemicals by electro-microfiltration, Environ. Sci. Technol., 2010, 44(20), 7914–7920 CrossRef CAS PubMed.
- Q. Yu, R. Zhang, S. Deng, J. Huang and G. Yu, Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: kinetic and isotherm study, Water Res., 2009, 43(4), 1150–1158 CrossRef CAS PubMed.
- X. Li, S. Chen, X. Quan and Y. Zhang, Enhanced adsorption of PFOA and PFOS on multiwalled carbon nanotubes under electrochemical assistance, Environ. Sci. Technol., 2011, 45(19), 8498–8505 CrossRef CAS PubMed.
- V. Ochoa-Herrera and R. Sierra-Alvarez, Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge, Chemosphere, 2008, 72(10), 1588–1593 CrossRef CAS PubMed.
- S. Deng, Q. Yu, J. Huang and G. Yu, Removal of perfluorooctane sulfonate from wastewater by anion exchange resins: effects of resin properties and solution chemistry, Water Res., 2010, 44(18), 5188–5195 CrossRef CAS PubMed.
- A. Urtiaga, C. Fernández-González, S. Gómez-Lavín and I. Ortiz, Kinetics of the electrochemical mineralization of perfluorooctanoic acid on ultrananocrystalline boron doped conductive diamond electrodes, Chemosphere, 2014, 129, 20–26 CrossRef PubMed.
- X. Zhu, M. Tong, S. Shi, H. Zhao and J. Ni, Essential explanation of the strong mineralization performance of boron-doped diamond electrodes, Environ. Sci. Technol., 2008, 42(13), 4914–4920 CrossRef CAS.
- Y. Q. Cong and Z. C. Wu, Electrocatalytic Generation of Radical Intermediates over Lead Dioxide Electrode Doped with Fluoride, J. Phys. Chem. C, 2007, 111(8), 3442–3446 CAS.
- L. L. Houk, S. K. Johnson and J. Feng, et al. Electrochemical incineration of benzoquinone in aqueous media using a quaternary metal oxide electrode in the absence of a soluble supporting electrolyte, J. Appl. Electrochem., 1998, 28(11), 1167–1177 CrossRef CAS.
- Z. Guohua, C. Xiao, L. Meichuan, L. Peiqiang, Z. Yonggang, C. Tongcheng, L. Hongxu, L. Yanzhu, L. Lei and L. Dongming, Electrochemical Degradation of Refractory Pollutant Using a Novel Microstructured TiO2 Nanotubes/Sb-Doped SnO2 Electrode, Environ. Sci. Technol., 2009, 43, 1480–1486 CrossRef.
- Y. Xiupei, Z. Ruyi, H. Feng, C. Duochang and X. Dan, Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol, J. Hazard. Mater., 2009, 164, 367–373 CrossRef PubMed.
- Q. Zhuo, S. Deng, B. Yang, J. Huang and G. Yu, Efficient electrochemical oxidation of perfluorooctanoate using a Ti/SnO2–Sb–Bi anode, Environ. Sci. Technol., 2011, 45(7), 2973–2979 CrossRef CAS PubMed.
- J. Niu, H. Lin, J. Xu, H. Wu and Y. Li, Electrochemical mineralization of perfluorocarboxylic acids (PFCAs) by Ce-doped modified porous nanocrystalline PbO2 film electrode, Environ. Sci. Technol., 2012, 46(18), 10191–10198 CAS.
- H. Lin, J. Niu, S. Ding and L. Zhang, Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes, Water Res., 2012, 46(7), 2281–2289 CrossRef CAS PubMed.
- H. Zhao, J. Gao, G. Zhao, J. Fan, Y. Wang and Y. Wang, Fabrication of novel SnO2–Sb/carbon aerogel electrode for ultrasonic electrochemical oxidation of perfluorooctanoate with high catalytic efficiency, Environ. Sci. Technol., 2013, 136, 278–286 Search PubMed.
- J. Niu, H. Lin, C. Gong and X. Sun, Theoretical and experimental insights into the electrochemical mineralization mechanism of perfluorooctanoic acid, Environ. Sci. Technol., 2013, 47(24), 14341–14349 CrossRef CAS PubMed.
- H. Lin, J. Niu, J. Xu, H. Huang, D. Li, Z. Yue and C. Feng, Highly Efficient and Mild Electrochemical Mineralization of Long-Chain Perfluorocarboxylic Acids (C9 and C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD Electrodes, Environ. Sci. Technol., 2013, 47(22), 13039–13046 CrossRef CAS PubMed.
- Z. Qiuming, Q. Yuqing, Z. Minshou and W. Limin, Structure and Electrochemical Properties of Yb-doped Lithium Iron Phosphate Cathode Materials, J. Chin. Soc. Rare Earths, 2012, 30(1), 78–85 Search PubMed.
- C. L. Heng, J. T. Li, W. Y. Su, Z. Han, P. G. Yin and T. G. Finstad, The formation of Yb silicates and its luminescence in Yb heavily doped silicon oxides after high temperature annealing, Opt. Mater., 2014, 42(1), 17–23 Search PubMed.
- L.-A. P. Thi, H.-T. Do, Y.-C. Lee and S.-L. Lo, Photochemical decomposition of perfluorooctanoic acids in aqueous carbonate solution with UV irradiation, Chem. Eng. J., 2013, 221, 258–263 CrossRef PubMed.
- M. Zhou, Q. Dai, L. Lei, C. a. Ma and D. Wang, Long life modified lead dioxide anode for organic wastewater treatment: electrochemical characteristics and degradation mechanism, Environ. Sci. Technol., 2005, 39(1), 363–370 CrossRef CAS.
- Y. Q. Cong and Z. C. Wu, Electrocatalytic Generation of Radical Intermediates over Lead Dioxide Electrode Doped with Fluoride, J. Phys. Chem. C, 2007, 111(8), 3442–3446 CAS.
- C. A. Martinez-huitle, M. A. Quiroz and C. Comninellis, et al. Electro-chemical incineration of chloranilic acid using Ti/IrO2, Pb/PbO2 and Si/BDD electrodes, Electrochim. Acta, 2004, 50(4), 949–956 CrossRef CAS PubMed.
- Q. Zhuo, S. Deng, B. Yang, J. Huang and G. Yu, Efficient electrochemical oxidation of perfluorooctanoate using a Ti/SnO2–Sb–Bi anode, Environ. Sci. Technol., 2011, 45(7), 2973–2979 CrossRef CAS PubMed.
- Q. Zhuo, S. Deng, B. Yang, J. Huang, B. Wang, T. Zhang and G. Yu, Degradation of perfluorinated compounds on a boron-doped diamond electrode, Electrochim. Acta, 2012, 77, 17–22 CrossRef CAS PubMed.
- Y.-C. Chen, S.-L. Lo and J. Kuo, Effects of titanate nanotubes synthesized by a microwave hydrothermal method on photocatalytic decomposition of perfluorooctanoic acid, Water Res., 2011, 45(14), 4131–4140 CrossRef CAS PubMed.
- D. C. Burns, D. A. Ellis, H. Li, C. J. McMurdo and E. Webster, Experimental pKa determination for perfluorooctanoic acid (PFOA) and the potential impact of pKa concentration dependence on laboratory-measured partitioning phenomena and environmental modeling, Environ. Sci. Technol., 2008, 42(24), 9283–9288 CrossRef CAS.
- R. Dillert, D. Bahnemann and H. Hidaka, Light-induced degradation of perfluorocarboxylic acids in the presence of titanium dioxide, Chemosphere, 2007, 67(4), 785–792 CrossRef CAS PubMed.
- D. A. Ellis, J. W. Martin, A. O. de Silva, S. A. Mabury, M. D. Hurley, M. P. Sulbaek Andersen and T. J. Wallington, Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids, Environ. Sci. Technol., 2004, 38(12), 3316–3321 CrossRef CAS.
- S. Kutsuna and H. Hori, Rate constants for aqueous-phase reactions of SO4− with C2F5C(O)O− and C3F7C(O)O− at 298 K, Int. J. Chem. Kinet., 2007, 39(5), 276–288 CrossRef CAS PubMed.
- C. Jing, P.-y. Zhang and L. Jian, Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light, J. Environ. Sci, 2007, 19(4), 387–390 CrossRef.
- C. Kormann, D. Bahnemann and M. R. Hoffmann, Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions, Environ. Sci. Technol., 1991, 25(3), 494–500 CrossRef CAS.
- S. C. Panchangam, A. Y.-C. Lin, K. L. Shaik and C.-F. Lin, Decomposition of perfluorocarboxylic acids (PFCAs) by heterogeneous photocatalysis in acidic aqueous medium, Chemosphere, 2009, 77(2), 242–248 CrossRef CAS PubMed.
- M. H. Plumlee, K. McNeill and M. Reinhard, Indirect photolysis of perfluorochemicals: hydroxyl radical-initiated oxidation of N-ethyl perfluorooctane sulfonamido acetate (N-EtFOSAA) and other perfluoroalkanesulfonamides, Environ. Sci. Technol., 2009, 43(10), 3662–3668 CrossRef CAS.
- T. Yamamoto, Y. Noma, S.-i. Sakai and Y. Shibata, Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol, Environ. Sci. Technol., 2007, 41(16), 5660–5665 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14299g |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb atomic ratio of 95
Sb atomic ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Next, 15 mL of concentrated HCl solution was added, and the resulting solution was diluted to 100 mL using iso-propanol. This solution was marked as solution A. For the Ti/SnO2–Sb–Yb anode, we added trace ytterbium nitrate to the iso-propanol solution (which contained dissolved Sn and Sb) at a Sn
5. Next, 15 mL of concentrated HCl solution was added, and the resulting solution was diluted to 100 mL using iso-propanol. This solution was marked as solution A. For the Ti/SnO2–Sb–Yb anode, we added trace ytterbium nitrate to the iso-propanol solution (which contained dissolved Sn and Sb) at a Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb atomic ratio of 95
Yb atomic ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. This solution was marked as solution B and was used to make the Ti/SnO2–Sb–Yb electrode only. We prepared three identical Ti sheets, of which two (marked as sheets 1 and 2, respectively) were dipped in solution A, while the other sheet (sheet 3) was dipped in solution B for 5 min. The sheets were then dried at 130 °C for 10 min, and the organics were thermally decomposed at 490 °C for 5 min. This dipping–annealing cycle was repeated 12 times. In the final stage, the coating was annealed for 1 h at 490 °C. Sheet 3 was the Ti/SnO2–Sb–Yb electrode. Electro-deposition was employed for the preparation of Ti/SnO2–Sb/Yb–PbO2 and Ti/SnO2–Sb–PbO2. Sheet 1 was placed into an acidic (pH 1, adjusted with HNO3) electro-deposition solution, which contained 200 g L−1 Pb(NO3)2, 2 g L−1 NaF, and 2.36 g L−1 Yb(NO3)3·6H2O at a constant anode current density of 20 mA cm−2 for 90 min under room temperature (25 °C). The anode was used as the Ti/SnO2–Sb/Yb–PbO2 electrode. Sheet 2 was treated similarly as sheet 1 except without the addition of Yb(NO3)3. The preparation of Ti/SnO2–Sb–PbO2 was thus completed.
0.5. This solution was marked as solution B and was used to make the Ti/SnO2–Sb–Yb electrode only. We prepared three identical Ti sheets, of which two (marked as sheets 1 and 2, respectively) were dipped in solution A, while the other sheet (sheet 3) was dipped in solution B for 5 min. The sheets were then dried at 130 °C for 10 min, and the organics were thermally decomposed at 490 °C for 5 min. This dipping–annealing cycle was repeated 12 times. In the final stage, the coating was annealed for 1 h at 490 °C. Sheet 3 was the Ti/SnO2–Sb–Yb electrode. Electro-deposition was employed for the preparation of Ti/SnO2–Sb/Yb–PbO2 and Ti/SnO2–Sb–PbO2. Sheet 1 was placed into an acidic (pH 1, adjusted with HNO3) electro-deposition solution, which contained 200 g L−1 Pb(NO3)2, 2 g L−1 NaF, and 2.36 g L−1 Yb(NO3)3·6H2O at a constant anode current density of 20 mA cm−2 for 90 min under room temperature (25 °C). The anode was used as the Ti/SnO2–Sb/Yb–PbO2 electrode. Sheet 2 was treated similarly as sheet 1 except without the addition of Yb(NO3)3. The preparation of Ti/SnO2–Sb–PbO2 was thus completed.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CF− and
CF− and  represents the slope of this line. The concentration of F− can be obtained from the standard curve of F− (Fig. S3†).
represents the slope of this line. The concentration of F− can be obtained from the standard curve of F− (Fig. S3†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 C mol−1), V is the volume of electrolyte (L), 8 is the equivalent mass of oxygen (g mol−1), I is the current (A), and t means the electrolysis time (s).
487 C mol−1), V is the volume of electrolyte (L), 8 is the equivalent mass of oxygen (g mol−1), I is the current (A), and t means the electrolysis time (s).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/C) of the three anodes were 0.0193, 0.012 and 0.011 min−1, respectively, and their defluorination ratios of PFOA were 76.7 ± 2.8%, 61.4 ± 1.1% and 52.8 ± 2.8%, respectively, as shown in Fig. 1. According to the rate constants, the degradation of the optimal reaction conditions (Table 1). A 200 mL PFOA solution was used in the electrochemical experiments to examine the effects of current density (1 mA cm−2 to 40 mA cm−2), initial concentration (10 mg L−1 to 200 mg L−1), electrode distance (5 mm to 20 mm), and initial pH (3 to 7).
C0/C) of the three anodes were 0.0193, 0.012 and 0.011 min−1, respectively, and their defluorination ratios of PFOA were 76.7 ± 2.8%, 61.4 ± 1.1% and 52.8 ± 2.8%, respectively, as shown in Fig. 1. According to the rate constants, the degradation of the optimal reaction conditions (Table 1). A 200 mL PFOA solution was used in the electrochemical experiments to examine the effects of current density (1 mA cm−2 to 40 mA cm−2), initial concentration (10 mg L−1 to 200 mg L−1), electrode distance (5 mm to 20 mm), and initial pH (3 to 7).







