Exploring the metallic phase of N2O under high pressure
Abstract
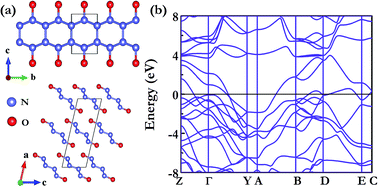
Simple molecular solids are expected to undergo structural phase transitions to form frameworks or polymeric solids under high pressure. The high-pressure structures of N2O have long attracted considerable attention. We combine the CALYPSO searching method with density functional theory to investigate the phase stabilities and structural changes of N2O at high pressure. We find two metallic structures of N2O which may be observed in high-pressure experiments. The current calculations reveal that the C2/m is the most stable structure over a pressure range of 177–194 GPa, and the other C2/c is metastable and only 10 meV per atom higher in energy than the C2/m structure at 180 GPa. At higher pressure, the metallic C2/m phase transforms into an insulating phase with space group of P21/m.

 Please wait while we load your content...
Please wait while we load your content...