Selective removal of thiosulfate from thiocyanate-containing water by a three-dimensional structured adsorbent: a calcined NiAl-layered double hydroxide film
Abstract
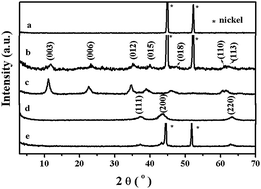
NiAl-layered double hydroxide (NiAl-LDH) platelets were uniformly grown on a porous Ni foam substrate by a facile in situ hydrothermal method. By subsequent calcination, the three-dimensional (3D) structured adsorbent, a calcined NiAl-layered double hydroxide film/Ni foam (NiAl-LDO/NF) was obtained. Batch experiments were carried out to investigate the selective adsorption performance of the obtained material for thiosulfate and thiocyanate anions in water, compared with the powder NiAl-LDO adsorbent. The results show that the NiAl-LDO/NF has highly selective adsorption for S2O32− in the mixture solution with a maximum adsorptive capacity of about 209.4 mg g−1 at room temperature, while the removal capacity for SCN− was only about 15.9 mg g−1. At the same time, the resultant 3D structured adsorbent exhibited higher preferential adsorption and easier separation performance from the solution than the corresponding NiAl-LDO powder. It was found that the adsorbability and selectivity of this material was well maintained after more than 10 regeneration cycles. Therefore, the obtained 3D hierarchical NiAl-LDO/NF can be considered as a potential structured adsorbent in environmental applications for the selective removal of S2O32− from SCN−-containing aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...