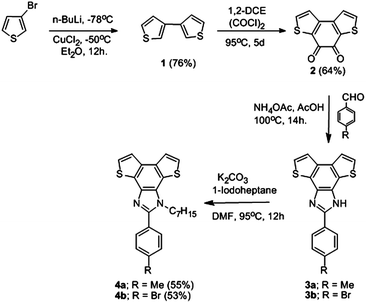
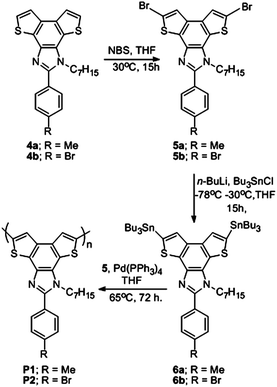
Benzodithieno-imidazole based π-conjugated fluorescent polymer probe for selective sensing of Cu2+†
Dipanjan Giri and
Sanjib K Patra*
Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur-721302, WB, India. E-mail: skpatra@chem.iitkgp.ernet.in; Tel: +91 3222283338
First published on 10th September 2015
Abstract
π-Conjugated polymers appended with binding sites are of considerable interest as an evolving new class of heavy and toxic metal ion sensors through fluorescence quenching. In this study we describe the synthesis and characterization of a p-bromophenyl substituted benzodithieno-imidazole based soluble and rigid π-conjugated polymer with N and S donors exhibiting outstanding sensitivity towards Cu2+ by emission quenching through photoinduced electron transfer (PET). Detailed photophysical and ion sensing studies have been demonstrated to understand insight of the polymer–metal ion interaction which is responsible for selective fluorescence quenching. The corresponding polymer is also explored as a thin-film polymeric sensor towards Cu2+ as monitored by a photoluminescence study.
Introduction
The development of highly sensitive and selective chemosensory materials for the detection of metal ions in environmental or biological systems has gained remarkable interest in recent years.1 Since heavy metal ions are significant pollutants and many of them are essential trace elements in biological systems, various fluorescence sensors of heavy metal ions have been developed for selective detection.2 For the detection of transition metal ions, binding sites are generated through the modification of the coordination sites through judicious design and synthesis.3Copper is the third most abundant transition metal ion found in all living organisms and is an essential trace element in redox chemistry. Several enzymes and proteins involved in metabolism, respiration, and DNA synthesis, such as cytochrome oxidase, superoxide dismutase, ascorbate oxidase, and tyrosinase, go through the copper cycle. The imbalance of copper in a living body results in various diseases such as Wilson disease (WD),4 Alzheimer’s disease,5 haematological manifestation and Menkes disease (MD).6 Therefore a reliable easy method for the detection of trace amounts of copper ions in biological and environmental samples is essential.
Many advanced methods such as spectrophotometry, atomic absorption spectroscopy, inductively coupled plasma mass spectroscopy, and conductometric detection have been developed for qualitative and quantitative detection of Cu2+ ions.7 Among them, due to the excellent sensitivity as well as selectivity, non-destructiveness, and economic nature, the fluorescence technique is the most used and is a rapidly expanding method in the field of chemical sensing.8 This process offers a highly sensitive optical transduction method for analyte binding events, which are based upon changes in intensity, energy transfer, wavelength shift (excitation and emission), optical changes and lifetime. The intramolecular charge transfer (ICT) and photoinduced electron transfer (PET) fluorescence mechanisms have been exploited to demonstrate the turn-on or turn-off fluorescence response during the sensing event by fluorescence sensors. In recent years many probes such as cyclen, imidazoquinoxaline, imidazopyrene, imidazophenazine, rhodamine, calixarene, bis(N-methylndolyl)methane, triazole based dansyl, dipicolylamine, 2,2′-dipicolylamine, and N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine based fluorescence sensors for heavy metal ions such as Zn2+, Pb2+, Hg2+, Cd2+, and Cu2+ have been successfully designed and developed with small molecule fluorophores as well as polymer probes by various groups.9
The field of π-conjugated polymer based metal ion sensors is of considerable interest and is of rapidly developing research interest with well defined systems via the fluorescence quenching mechanism. Thus the desired polymer regime can be integrated with the incorporation of the main-chain or side-chain with strong binding sites. The delocalized π-electronic conjugated polymer is a prominent candidate for sensitivity enhancement in fluorescence quenching processes due to the fast and facile energy migration along the π-conjugated chain, resulting in optical signals for transduction of chemical or biological events as demonstrated by various groups.10 In spite of wide research in this field, it remains largely unknown how the structural modification on the coordination site and the substituent helps the sensing efficacy. We are interested in investigating the structural modification of the fluorophores through the rational design of molecular architectures searching for an efficient polymer probe for metal ions. In this work, we report benzothieno-imidazole based π-conjugated luminescent polymeric probes having nitrogen and sulphur donors acting as chelating sites for selective sensing of metal ions. Lin and co-workers first showed that the benzothieno-imidazole moiety can be used as a good sensory probe for toxic heavy metal cations.11 Furthermore the presence of both hard N and soft S donors could give an efficient fluorescence sensor for selective transition metal cations having a borderline acidic nature such as Cu2+. The corresponding polymer is also investigated for sensory applications towards Cu2+ as a thin-film polymeric sensor as monitored by a photoluminescence (PL) study.
Results and discussion
Synthesis and characterization
A good chelating or a binding site is necessary to improve the binding constant as well as to enhance the fluorescence response.12 For this reason the benzodithieno-imidazole based ligand containing the two donor hetero-atoms, nitrogen and sulphur, first introduced by Lin and co-workers11 has been explored in this work. The key precursor of this work, 3,3′-bithiophene (1) was synthesized from 3-bromothiophene by treatment with n-BuLi at −78 °C followed by anhydrous CuCl2 at −50 °C in diethyl ether, through Gilman coupling. The resulting white solid was purified through column chromatography using hexane as the eluent to get 1 in a 76% yield. 1H NMR shows two multiplets, one at 7.37–7.38 ppm for the two protons adjacent to the sulphur (5 and 5′ position) atom and another multiplet at 7.36–7.33 ppm for the remaining four thienyl protons. Acylation of 1 by oxalyl chloride in 1,2-DCE at 95 °C yielded 2 as a shiny reddish solid with a 64% yield. In 1H NMR the signal at 7.83 ppm signifies two more deshielded protons adjacent to the sulphur atom, and the resonance at 7.29 ppm is attributed to the other two less deshielded protons. Compound 2 was coupled with 4-substituted benzaldehydes (4-methyl benzaldehyde and 4-bromo benzaldehyde) as shown in Scheme 1, in the presence of ammonium acetate in acetic acid medium to get the compounds 3a and 3b respectively as greenish solids. As these compounds are insoluble in common organic solvents, alkylation is necessary to improve the solubility. For this N-alkylation was performed by treatment with potassium carbonate and n-iodoheptane. The compound was purified through column chromatography using EtOAc–hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to obtain the analytically pure yellow solid of 4a (p-tolylbenzodithieno-imidazole) and 4b (p-bromophenylbenzodithieno-imidazole) in a 55% and 53% yield respectively. The formation of 4a was confirmed by a 1H NMR spectrum in CDCl3, showing two doublets at 7.67 ppm and 7.36 ppm corresponding to the four thiophene protons. The two benzene protons (ortho to Me) resonate as a triplet centred at 7.52 ppm whereas the other two protons resonate as a doublet of doublets at 7.80 and 7.87 ppm. The methyl protons appear at 2.46 ppm as a singlet. The characteristic peak at 4.51 ppm represents the two methylene protons attached to the N atom. For 4b the signals at 7.83 ppm and 7.60 ppm signify the four thiophene protons, whereas the four benzene protons resonate at 7.73 ppm as a multiplet and at 7.51 ppm as a doublet. The HRMS (ESI+) study also confirms the formation of products 4a and 4b by showing the molecular ion peak at 419.1599 ([M + H]+) and 483.3438 ([M + H]+) respectively. For 4b the yellow needle-like single crystals suitable for X-ray crystallography were harvested by layering a THF solution of 4b on water. 4b crystallizes in a triclinic system with the space group P
1) as the eluent to obtain the analytically pure yellow solid of 4a (p-tolylbenzodithieno-imidazole) and 4b (p-bromophenylbenzodithieno-imidazole) in a 55% and 53% yield respectively. The formation of 4a was confirmed by a 1H NMR spectrum in CDCl3, showing two doublets at 7.67 ppm and 7.36 ppm corresponding to the four thiophene protons. The two benzene protons (ortho to Me) resonate as a triplet centred at 7.52 ppm whereas the other two protons resonate as a doublet of doublets at 7.80 and 7.87 ppm. The methyl protons appear at 2.46 ppm as a singlet. The characteristic peak at 4.51 ppm represents the two methylene protons attached to the N atom. For 4b the signals at 7.83 ppm and 7.60 ppm signify the four thiophene protons, whereas the four benzene protons resonate at 7.73 ppm as a multiplet and at 7.51 ppm as a doublet. The HRMS (ESI+) study also confirms the formation of products 4a and 4b by showing the molecular ion peak at 419.1599 ([M + H]+) and 483.3438 ([M + H]+) respectively. For 4b the yellow needle-like single crystals suitable for X-ray crystallography were harvested by layering a THF solution of 4b on water. 4b crystallizes in a triclinic system with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The crystal structure of 4b consists of two independent molecules in an asymmetric unit with negligible differences in metrical parameters. The molecular structure (ORTEP representation) of one of the molecules in the asymmetric unit is shown in Fig. 1. The X-ray study reveals the planar conformation of the benzodithieneo-imodazole core confirming the higher degree of π-electron delocalization which is also reflected in the photophysical properties of 4b and the corresponding π-conjugated rigid polymer, P2 (vide infra). The phenyl and the benzodithieno-imidazole rings are nearly orthogonal to each other as manifested by the torsion angle (C5–C4–C7–N1) of 83.8°.
. The crystal structure of 4b consists of two independent molecules in an asymmetric unit with negligible differences in metrical parameters. The molecular structure (ORTEP representation) of one of the molecules in the asymmetric unit is shown in Fig. 1. The X-ray study reveals the planar conformation of the benzodithieneo-imodazole core confirming the higher degree of π-electron delocalization which is also reflected in the photophysical properties of 4b and the corresponding π-conjugated rigid polymer, P2 (vide infra). The phenyl and the benzodithieno-imidazole rings are nearly orthogonal to each other as manifested by the torsion angle (C5–C4–C7–N1) of 83.8°.
To access the corresponding polymers of 4a and 4b through step growth polymerization, the respective monomers 5a and 5b were prepared through bromination using N-bromosuccinamide (NBS) in an 80–85% yield.11a However Grignard Reagent Metathesis (GRIM)13 was unsuccessful in the presence of Ni(dppp)Cl2 (dppp = 1,3-bis(diphenylphosphino)propane) in combination with the various Grignard reagents such as t-BuMgCl, i-PrMgBr, cyclohexylMgBr and turbo Grignard (i-PrMgCl·LiCl) reagents under various reaction conditions (see Table S2†). The Stille polymerization protocol of 5a and 5b was followed to obtain the corresponding π-conjugated polymers as shown in Scheme 2. Addition of n-butyllithium followed by tributyltinchloride at −78 °C to 5a and 5b afforded the respective bis(tributylstannyl) derivatives 6a and 6b, which were used directly for Stille polymerization.14 Polymerization was performed with 5a and 6a in the presence of a Pd(PPh3)4 catalyst through the Stille polymerization protocol15 to get P1 (Scheme 2). In a similar strategy P2 was also synthesized. The polymers were isolated through precipitation in methanol. The polymer was further purified through a Soxhlet extractor using hexane, then MeOH, and finally the polymer was extracted with DCM. Then the DCM solution was concentrated and precipitated in stirring distilled hexane. The precipitate was washed four times with hexane to get the desired brownish coloured polymer. The polymerization conditions and characterization data are shown in Table 1. The polymers were found to be readily soluble in common organic solvents and exhibited a strong cyan fluorescence. The luminescent π-conjugated polymers were characterized by various spectroscopic tools. The disappearance of the alkyl protons of SnBu3 in 1H NMR and the disappearance of the signal at 112.2 and 111.5 ppm in 13C NMR corresponding to the thienyl carbons attached to Br reveal polymerization of 5b and 6b. The formation of the polymer was further confirmed by Tetradetector Gel Permeation Chromatography (GPC) as shown in Fig. 2. The molecular weight distribution with a PDI of 1.81 and 1.67 was observed for P1 and P2 respectively indicating the step growth polymerization of the corresponding monomers.
| Polymer | Yield | Mn (Da) | PDI (Mw/Mn) |
|---|---|---|---|
| a Stille polymerization of 5a and 5b in THF was carried out at 65 °C for 72 h using Pd(PPh3)4 as the catalyst (5 mol%). | |||
| P1 | 65% | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799 799 |
1.81 |
| P2 | 68% | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 519 |
1.67 |
 | ||
| Fig. 2 GPC trace (refractive index and UV responses) of P2 using THF as the eluent. The PDI (Mw/Mn) of P2 was found to be 1.67. | ||
Photophysical studies
The absorption studies of 4a and 4b in DMSO show two absorption maxima, one at high energy centred at 270 nm and the second one with low energy in the range of 320–350 nm. The low energy absorption bands (333 nm for 4a and 340 nm for 4b) are assigned to the π–π* transition involving the molecular orbital of the benzodithieno and imidazole moieties. The fluorescence response of the fluorophores 4a and 4b was explored in DMSO (Fig. S36†) exhibiting a strong blue fluorescence centred at 407 (λexct = 333 nm) and 422 (λexct = 340 nm) nm respectively. The low energy absorption for both the polymers is red shifted by 55–60 nm compared to that of the corresponding monomers, suggesting extensive π-delocalization through the polymer backbone (Fig. S51†). The fluorescence response of P1 and P2 was investigated (in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) showing cyan emission centred at 460 nm and 468 nm respectively which was again 40–60 nm red shifted from the blue emission of 4a and 4b as shown in Fig. 3. The quantum yield (ϕf) of emission of 4a and 4b and the corresponding polymers P1 and P2 in DMSO
H2O) showing cyan emission centred at 460 nm and 468 nm respectively which was again 40–60 nm red shifted from the blue emission of 4a and 4b as shown in Fig. 3. The quantum yield (ϕf) of emission of 4a and 4b and the corresponding polymers P1 and P2 in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was 0.19, 0.09, 0.21 and 0.23 respectively (Table 2) with reference to quinine sulphate in 0.1 M H2SO4 solution which was calculated according the literature procedure as described in the ESI.†
1) was 0.19, 0.09, 0.21 and 0.23 respectively (Table 2) with reference to quinine sulphate in 0.1 M H2SO4 solution which was calculated according the literature procedure as described in the ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)
| Fluorophore | Absorbance | Luminescence | |||
|---|---|---|---|---|---|
| λmax (nm) | ε (L mol−1 cm−1) × 102 | λexct (nm) | λem (nm) | ϕfa | |
| a Quinine sulphate in 0.1 M H2SO4 was used as a reference for the quantum yield calculation with ϕf = 0.57. | |||||
| 4a | 308, 333 | 198, 71 | 333 | 407 | 0.19 |
| 4b | 270, 340 | 196, 78 | 340 | 422 | 0.09 |
| P1 | 279, 395 | 273, 226 | 395 | 460 | 0.21 |
| P2 | 279, 400 | 298, 185 | 400 | 468 | 0.23 |
Metal ion sensing properties
Before studying metal ion complexation of polymers for demonstrating sensory properties towards transition metal cations, we investigated the metal coordination with the corresponding monomers searching for an efficient and selective sensor for Cu2+. After complete characterization the sensing ability of fluorophores 4a and 4b was verified with different metal cations. Thus the absorbance and fluorescence response of 4a and 4b for different metal ions such as Co2+, Cu2+, Ni2+, Zn2+, Cd2+, Pb2+, Cr3+, Fe3+, Hg2+, Ba2+ and Sr2+ was investigated in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O to examine the selective response to Cu2+. The fluorophore and the metal ions were dissolved in DMSO and water respectively. The solutions of these metal cations were prepared in water in ∼1 × 10−5 M concentration and were added to the stock solution of 4a and 4b which was also prepared in ∼1 × 10−5 M concentration in DMSO by maintaining the 1
H2O to examine the selective response to Cu2+. The fluorophore and the metal ions were dissolved in DMSO and water respectively. The solutions of these metal cations were prepared in water in ∼1 × 10−5 M concentration and were added to the stock solution of 4a and 4b which was also prepared in ∼1 × 10−5 M concentration in DMSO by maintaining the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal to ligand ratio. The fluorophore 4a with the electron donating Me substituted congener does not show any significant change in absorption or in PL intensity upon addition of different metal cations (Fig. S37 and S38†). Interestingly 4b with an electron withdrawing Br substituent shows a significant photophysical change upon addition of Cu2+ ions indicating the preferential selectivity towards Cu2+ among various competing metal cations. Considering the absorption spectrum of 4b as shown in Fig. S39,† the absorption maxima of the free ligand were observed at 270 nm and 340 nm. The absorption was due to the π–π* transition involving the imidazole and dithieno moieties. On addition of different metal ions the absorption maxima blue shifted by ca. 10 nm compared to the absorption of the free ligand. Surprisingly after addition of Cu2+ the absorption maximum at 340 nm (corresponding to the transition of π to π*) was diminished and a new absorption maximum at 286 nm appeared, indicating the coordination of Cu2+ to the binding sites of 4b. The absorption study clearly shows that the fluorophore 4b can selectively bind to Cu2+, which is further demonstrated by fluorescence quenching after coordination to paramagnetic Cu2+ selectively as demonstrated in Fig. 4.
1 metal to ligand ratio. The fluorophore 4a with the electron donating Me substituted congener does not show any significant change in absorption or in PL intensity upon addition of different metal cations (Fig. S37 and S38†). Interestingly 4b with an electron withdrawing Br substituent shows a significant photophysical change upon addition of Cu2+ ions indicating the preferential selectivity towards Cu2+ among various competing metal cations. Considering the absorption spectrum of 4b as shown in Fig. S39,† the absorption maxima of the free ligand were observed at 270 nm and 340 nm. The absorption was due to the π–π* transition involving the imidazole and dithieno moieties. On addition of different metal ions the absorption maxima blue shifted by ca. 10 nm compared to the absorption of the free ligand. Surprisingly after addition of Cu2+ the absorption maximum at 340 nm (corresponding to the transition of π to π*) was diminished and a new absorption maximum at 286 nm appeared, indicating the coordination of Cu2+ to the binding sites of 4b. The absorption study clearly shows that the fluorophore 4b can selectively bind to Cu2+, which is further demonstrated by fluorescence quenching after coordination to paramagnetic Cu2+ selectively as demonstrated in Fig. 4.
 | ||
Fig. 4 Emission spectra of 4b (∼2 × 10−5 M) in the presence of different metal ions in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
To study the sensing in more depth and to ensure the phenomenon, a titration of ligand 4b with Cu2+ solution was performed with increasing concentrations of Cu2+ and the UV-vis absorption (Fig. S43†) as well as emission (Fig. S44†) spectral responses were recorded. The absorption maximum at 340 nm corresponding to the free ligand decreases upon increasing the concentration of Cu2+, probably due to the gradual diminishing of the π–π* transition from imidazole to benzodithieno as a result of the adduct formation with Cu2+. The isosbestic point at 327 nm revealed the existence of a single equilibrium between the free ligand and the Cu2+ bound ligand. The emission spectrum shows a gradual quenching upon increasing the Cu2+ concentration and at a higher concentration (2 × 10−4 M) of Cu2+ the fluorescence was completely quenched. Saturation of the PL intensity was observed on addition of 0.6 equiv. of Cu2+ suggesting a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (4b·Cu2+) stoichiometry. This was further confirmed by the continuous variation method (Job’s plot) as depicted in Fig. 5. The intensity at 422 nm was plotted against the mole fraction of Cu2+ at a constant total concentration ([Cu2+]·4b) of 20 mM and the spectra were acquired in DMSO
1 (4b·Cu2+) stoichiometry. This was further confirmed by the continuous variation method (Job’s plot) as depicted in Fig. 5. The intensity at 422 nm was plotted against the mole fraction of Cu2+ at a constant total concentration ([Cu2+]·4b) of 20 mM and the spectra were acquired in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The association constant of Cu2+ with 4b was found to be 5.2 (±0.2) × 104 indicating thermodynamically stable binding between the borderline Cu2+ and the 4b ligand containing N and S donors, which can be studied by the Stern–Volmer10a,16 equation (F0/F = (1 + KSV[Q])) (Fig. S45†). The 4b·Cu2+ metal complex was isolated by reacting 4b and Cu(NO3)2 in a THF–methanol mixture. The MALDI-TOF study confirms the 2
1). The association constant of Cu2+ with 4b was found to be 5.2 (±0.2) × 104 indicating thermodynamically stable binding between the borderline Cu2+ and the 4b ligand containing N and S donors, which can be studied by the Stern–Volmer10a,16 equation (F0/F = (1 + KSV[Q])) (Fig. S45†). The 4b·Cu2+ metal complex was isolated by reacting 4b and Cu(NO3)2 in a THF–methanol mixture. The MALDI-TOF study confirms the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 4b·Cu2+ complex by showing a molecular ion peak at 1029.700 as observed by photophysical studies. The isotopic mass distribution of the complex (4b·Cu2+) with the proposed structure of the metal complex is shown in Fig. 6.
1 4b·Cu2+ complex by showing a molecular ion peak at 1029.700 as observed by photophysical studies. The isotopic mass distribution of the complex (4b·Cu2+) with the proposed structure of the metal complex is shown in Fig. 6.
 | ||
Fig. 5 Job’s plot showing 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between 4b and Cu2+ in DMSO 1 binding stoichiometry between 4b and Cu2+ in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. Plot recorded from (a) absorbance titration, and (b) emission titration. H2O. Plot recorded from (a) absorbance titration, and (b) emission titration. | ||
 | ||
| Fig. 6 (a) Simulated and (b) experimental isotopic distribution of (4b)2·Cu2+, with the proposed schematic representation of the binding sites where the Cu2+ metal ion is bound to 4b. | ||
One of the most important criterion for a selective cation probe is the ability to sense a particular cation in the presence of other competitive and similar cations. To show the selectivity towards Cu2+, the florescence intensity measurement of P2 was carried out in the presence of Cu2+ ions mixed with other metal cations such as Co2+, Ni2+, Zn2+, Cd2+, Pb2+, Cr3+, Fe3+, Hg2+, Ba2+ and Sr2+. It was observed to have excellent selectivity towards Cu2+ without showing any interference in the vicinity of other metal cations as shown in Fig. 7.
After the successful sensing study of 4b towards Cu2+ selectively, we were encouraged to explore the corresponding π-conjugated polymer as a probe for Cu2+ aiming for thin-film metal ion sensing. The polymer P2 also shows selective sensing towards Cu2+ as observed for the monomer as monitored by the quenching in emission in the presence of Cu2+. The selectivity towards Cu2+ in the presence of other ions (Fig. 8) of the polymer was performed, which again shows that the polymer can be used as a selective Cu2+ sensor. The visually noticeable fluorescence quenching with the naked eye (under UV illumination at 365 nm) makes P2 an efficient sensor for Cu2+ as demonstrated in Fig. 9. The absorption and fluorescence titration by varying the amount of Cu2+ was also performed showing significant change in the photophysical properties. The solutions of these metal salts were prepared in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at ∼2 × 10−5 M concentration and were added to the stock solution of polymer P2. A similar protocol for the absorption and emission titration was followed as performed in the case of 4b. From the titration profile it is obvious that the absorption maxima at 400 nm (Fig. 10) of the polymer decrease while λmax at 279 nm increases with the increasing concentration of Cu2+ accompanied by an isosbestic point at 348 nm suggesting a single equilibrium between the free polymer and the Cu2+-coordinated metallopolymer. Gradual quenching of emission with the increasing concentration of Cu2+ (Fig. 11) was observed as expected. However the complete quenching of fluorescence of the polymer backbone was not observed by the ligand–metal ion interaction unlike the monomer where complete quenching of fluorescence was observed, presumably due to the unavailability of all the coordination sites to metal ions in the polymer backbone.12a,17 It is noteworthy to mention that the limit of detection of P2 and 4b for Cu2+ (5.29 × 10−7 M−1 and 6.53 × 10−7 M−1 respectively) is sufficiently below the limit of copper in drinking water (20 μM) as calculated from the concentration dependent fluorescence studies shown in Fig. S52.†
1) at ∼2 × 10−5 M concentration and were added to the stock solution of polymer P2. A similar protocol for the absorption and emission titration was followed as performed in the case of 4b. From the titration profile it is obvious that the absorption maxima at 400 nm (Fig. 10) of the polymer decrease while λmax at 279 nm increases with the increasing concentration of Cu2+ accompanied by an isosbestic point at 348 nm suggesting a single equilibrium between the free polymer and the Cu2+-coordinated metallopolymer. Gradual quenching of emission with the increasing concentration of Cu2+ (Fig. 11) was observed as expected. However the complete quenching of fluorescence of the polymer backbone was not observed by the ligand–metal ion interaction unlike the monomer where complete quenching of fluorescence was observed, presumably due to the unavailability of all the coordination sites to metal ions in the polymer backbone.12a,17 It is noteworthy to mention that the limit of detection of P2 and 4b for Cu2+ (5.29 × 10−7 M−1 and 6.53 × 10−7 M−1 respectively) is sufficiently below the limit of copper in drinking water (20 μM) as calculated from the concentration dependent fluorescence studies shown in Fig. S52.†
 | ||
| Fig. 9 Visual changes observed in emission of free P2 (left) and Cu2+-coordinated P2·Cu2+ (right) under UV illumination at 365 nm. | ||
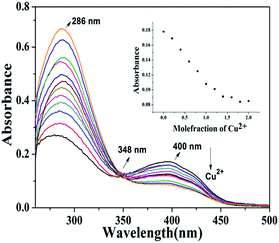 | ||
Fig. 10 Absorbance spectral changes of P2 (∼2 × 10−5 M) in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of different equivalents of Cu2+. Inset: absorbance as a function of [Cu2+]. 1) in the presence of different equivalents of Cu2+. Inset: absorbance as a function of [Cu2+]. | ||
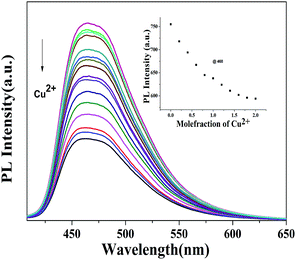 | ||
| Fig. 11 Changes in emission intensity of P2 (∼2 × 10−5 M) in DMSO : H2O upon addition of increasing equivalents of Cu2+. Inset: emission intensity as a function of [Cu2+]. | ||
Thus after coordination to paramagnetic Cu2+ ions, emission at 468 nm of P2 was quenched which was not observed in the case of other competing metal cations. Probably the soft and hard binding sites associated with the sulphur and nitrogen atoms can bind preferentially to the borderline acid Cu2+. The emission quenching phenomenon is generally observed from the three major mechanisms of fluorescence transduction such as intramolecular charge transfer (ICT),18 fluorescence resonance energy transfer (FRET),19 and photoinduced electron transfer (PET).1e,20 FRET is ruled out considering the negligible overlap region between the absorption and emission of the fluorophore unit. The absence of a separate interacting and proximal donor–acceptor pair in this system further excludes the possibility of FRET. ICT is also unlikely as there is no possibility of formation of an ICT structure in this probe. The emission quenching of 4b and the π-conjugated polymer (P2) after Cu2+ coordination is mainly due to the photoinduced electron transfer interaction between the fluorophore unit and paramagnetic Cu2+ as reported in the previous literature.21 In our case, upon binding the ligand with Cu2+ there is a large quenching of fluorescence intensity as reflected by the very low quantum yield (ϕ = 0.002). We propose that the chelating sites with the N and S hetero-atoms are responsible for binding with metal cations. However 4a and P1 did not show any change in PL intensity after addition of Cu2+ (vide supra). To understand the different photophysical response of 4a and 4b towards Cu2+ we calculated the HOMO and LUMO of the fluorophores by electrochemical studies (ESI†). Presumably the dx2−y2 orbital accommodating the unpaired electron of the paramagnetic Cu2+ (elongated octahedron) lies between the HOMO and LUMO of 4b. The successful recognition of Cu2+ by 4b through the possible PET mechanism has been demonstrated in Fig. S54a† showing a favourable energy difference between the LUMO (of 4b) and dx2−y2 (of Cu2+) leading to non-radiative deactivation of the excited state resulting in PL quenching.21,22 The LUMO of 4a (−3.02 eV) is significantly lower in energy than that of 4b (−2.71 eV), and also presumably lower in energy than the dx2−y2 orbital. Hence the electron transfer is not facile from the LUMO of 4a to dx2−y2 of Cu2+ (Fig. S54b†). The proposed phenomenon was further supported by the fluorescence decay study (Fig. 12) through Time-Correlated Single Photon Counting (TCSPC). In the fluorescence lifetime decay experiment (λem = 400 nm), the lifetime of free 4b was found to be 1.88 ns whereas for 4b·Cu2 the lifetime was decreased to 1.64 ns. For P2 (λem = 468 nm) the first lifetime component (τ1) was 2.55 (67%) with a second component of 3.43 ns (33%), and an average lifetime (τ) of 2.83 ns. The average fluorescence lifetime of the corresponding Cu2+-coordinated metal-containing polymer (P2·Cu2+) decreased to 2.34 ns (Table 3) ascribing the collisional fluorescence quenching phenomenon.23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)a,b,c
1)a,b,c
The Cu2+-coordinated complex 4b, [Cu(4b)2](NO3)2 and the corresponding metallopolymer (P2·Cu2+) were also characterized by a cyclic voltammetry study (Fig. 13). While recording CV at the window of +1.50 V to −1.50 V in DMF with a scan rate of 100 mV s−1 two reduction peaks at −0.446 V and −0.943 V attributed to the Cu2+/Cu1+ and Cu1+/Cu0 reduction processes were observed. The significantly intense anodic peak at +0.328 V is due to the deposition of Cu metal at the electrode surface as a result of Cu1+/Cu0 reduction.3h,24 A similar behaviour was also observed for the Cu2+-coordinated metallopolymer where a less intense anodic peak at +0.318 V was observed presumably due to the lower amount of Cu deposition at the electrode surface in the presence of polymers.
To demonstrate the practical application of on-site detection of Cu2+ by the polymer, we explored the detection by making a polymeric film on a quartz plate. A thin-film of the polymer was prepared by spin coating on the quartz plate, which was then immersed to various concentrations of Cu2+ aqueous solutions (1 mM to 1 μM) followed by repetitive and extensive washing with Milli-Q water to remove any unbound free Cu2+ ions. A solid state PL study of the Cu2+-coordinated polymeric film was performed to demonstrate a significant amount of quenching of the emission centred at 525 nm (Fig. 14). Energy Dispersive X-ray (EDX) analysis shows the presence of Cu confirming the formation of the Cu2+-coordinated metallopolymer. We also performed gradual quenching of the PL intensity with the increasing time of immersion of the polymeric film in Cu2+ solution (1 μM). It was observed that upon instant dipping of the quartz plate in the Cu2+ solution there is no such quenching. However if we allow for a longer time of 15 minutes or more in Cu2+ solution, the intensity of the PL spectra started to decrease significantly as shown in Fig. S56,† demonstrating that the polymer can be explored as a thin-film sensor for Cu2+ ion detection.
Conclusions
In conclusion, soluble benzodithieno-imidazole based π-conjugated rigid polymers, appended with N and S donors, were synthesized through Stille coupling searching for an efficient polymer probe for biological and environmentally relevant Cu2+. It is observed that the sensing ability is highly dependent on the substituent as revealed in this study. The electron withdrawing Br substituted p-bromophenylbenzodithieno-imidazole succeeds over the electron donating Me substituted p-tolylbenzodithieno-imidazole congener towards Cu2+ ion sensing. Thus the synthesized π-conjugated polymer P2 is shown to be an outstanding fluorescence sensor for the recognition of paramagnetic Cu2+ with high sensitivity and selectivity through fluorescence quenching via photoinduced electron transfer. Its fluorescence intensity decreases in a linear fashion with the concentration of Cu2+, demonstrating it to be a potential candidate for selective sensing of Cu2+ in the presence of different metal ions. The detailed photophysical and ion sensing including electrochemical studies were demonstrated to understand insight of the polymer–metal ion interaction. Furthermore the metal ion sensing ability by a polymeric film of P2 is examined to demonstrate the practical application in on-site detection of Cu2+. This work reveals a novel platform for the further development of soluble π-conjugated fluorescent polymer based probes by fine tuning of the electronic factors for sensing environmentally relevant metal ions.Experimental section
Materials and instrumentation
All the air and moisture sensitive reactions and manipulations were carried out under an atmosphere of pre-purified N2 or Ar using standard dual manifold Schlenk techniques. The glassware was oven dried (at 180 °C) and cooled under vacuum. Tetrahydrofuran and diethyl ether were dried over Na/benzophenone. All chemicals were purchased from Aldrich unless otherwise noted. Ammonium acetate and all metal salts were acquired from Merck. The benzaldehyde derivatives were purchased from Spectrochem. Silica gel (60–120 and 100–200 mesh) used for column chromatography was purchased from Merck. Pd(PPh3)4 was synthesized following the literature method.251H (600 MHz, 400 MHz and 200 MHz), 13C{1H} (150 MHz, 100 MHz and 50 MHz) NMR spectra were obtained from a Bruker Lambda spectrometer using CDCl3 unless otherwise mentioned. The spectra were internally referenced to residual solvent peaks (δ = 7.26 ppm for proton and δ = 77.23 for carbon (middle peak) in CDCl3). All coupling constants (J) are given in Hz. HRMS was recorded in ESI+ mode (70 eV) in a Waters mass spectrometer (Model: Xevo-G2QTOF). The absorption and fluorescence spectra were collected using a Shimadzu (Model UV-2450) spectrophotometer and a Hitachi (Model F-7000) spectrofluorimeter, respectively. FTIR spectroscopy was recorded in Spectrum-BX (Perkin Elmer). Solid state PL spectra were recorded in Flurolog Horiba (Model FL-1016, Spectracq). The MALDI-TOF study was performed using a Bruker MALDI-TOF-TOF-UltrafleXtreme instrument. Single-crystal data was collected in low temperature mode using a Bruker Apex-II instrument. The morphology and EDX analysis of the polymer thin-films were carried out using a JEOL JSM5800 (Japan) Scanning Electron Microscope with an Oxford EDS detector. The molecular weights and polydispersity indices (PDI = Mw/Mn) of the polymers were obtained by Gel Permeation Chromatography (GPC) using a Viscotek VE 2001 Triple-Detector Gel Permeation Chromatograph equipped with an automatic sampler, pump, injector, inline degasser, column oven (30 °C), styrene/divinylbenzene columns with pore sizes of 500 Å and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å, VE 3580 refractometer, four-capillary differential viscometer and 90° angle laser and low angle laser (7°) light scattering detector (VE 3210 & VE270). HPLC grade THF was used as the chromatography eluent, at a flow rate of 1.0 mL min−1. Samples were dissolved in the eluent (1 mg mL−1) and filtered with a Ministart SRP 15 filter (polytetrafluoroethylene membrane of 0.45 μm pore size) before analysis. Calibration of all three detectors (refractive index, laser light scattering and viscometry) was performed using polystyrene standards (Viscotek). This equipment allows the absolute measurement of homopolymer molecular weights and PDIs. Cyclic voltammetric studies were performed on a BASi Epsilon electrochemical workstation in acetonitrile with 0.1 M tetra-n-butylammoniumhexafluorophosphate (TBAPF6) as the supporting electrolyte at room temperature. The working electrode was a BASi Pt disc electrode, the reference electrode was Ag/AgCl and the auxiliary electrode was a Pt wire. The ferrocene/ferrocenium couple occurs at E1/2 = +0.51 (70) V versus Ag/AgCl under the same experimental conditions.
000 Å, VE 3580 refractometer, four-capillary differential viscometer and 90° angle laser and low angle laser (7°) light scattering detector (VE 3210 & VE270). HPLC grade THF was used as the chromatography eluent, at a flow rate of 1.0 mL min−1. Samples were dissolved in the eluent (1 mg mL−1) and filtered with a Ministart SRP 15 filter (polytetrafluoroethylene membrane of 0.45 μm pore size) before analysis. Calibration of all three detectors (refractive index, laser light scattering and viscometry) was performed using polystyrene standards (Viscotek). This equipment allows the absolute measurement of homopolymer molecular weights and PDIs. Cyclic voltammetric studies were performed on a BASi Epsilon electrochemical workstation in acetonitrile with 0.1 M tetra-n-butylammoniumhexafluorophosphate (TBAPF6) as the supporting electrolyte at room temperature. The working electrode was a BASi Pt disc electrode, the reference electrode was Ag/AgCl and the auxiliary electrode was a Pt wire. The ferrocene/ferrocenium couple occurs at E1/2 = +0.51 (70) V versus Ag/AgCl under the same experimental conditions.
3,3′-Bithiophene
In a 250 mL Schlenk flask 3-bromothiophene (5 g, 30.6 mmol) was dissolved in 100 mL anhydrous diethyl ether. The solution was cooled to −78 °C. To it n-BuLi in hexane (1.6 M in hexane, 20 mL, 33 mmol) was added dropwise for 40 min under Ar. The reaction was allowed to stir for 10 min at −78 °C and for 20 min at −60 °C. Then, CuCl2 (4.38 g, 32.5 mmol) was added in one portion at −60 °C. The reaction was carefully maintained at −60 °C for another 1 h. Then, the reaction mixture was slowly warmed to room temperature and continued stirring for another 18 h at RT. The reaction mixture was quenched by 30 mL water and filtered to remove inorganic impurities. The organic phase was collected and dried over anhydrous MgSO4. The solvent was removed by rotary evaporation. The crude product was purified by silica gel column chromatography using hexane as an eluent to get 1.9 g (76% yield) of 3,3′-bithiophene as a white crystalline solid. 1H NMR (200 MHz, CDCl3): δ (ppm) 7.37–7.38 (m, 2H), 7.33–7.36 (m, 4H). 13C{1H} NMR (50 MHz, CDCl3): δ (ppm) 137.4, 126.5, 126.2, 119.9.Benzo[1,2-b:4,3-b]dithiophene-4,5-quinone (2)
3,3′-Bithiophene (1.5 g, 9 mmol) and 1,2-dichloroethane (DCE) (30 mL) were added to a 250 mL two neck round bottom flask under Ar. Oxalyl chloride (1.4 g, 9 mmol) was added gradually (over 10 min), and the reaction mixture was stirred at 90 °C for 4 days. The reaction mixture was then cooled and kept at 0 °C overnight. It was filtered. The product was washed thoroughly by hexane. After drying, the product was obtained as a red solid (1.2 g, 64% yield). 1H NMR (400 MHz, CDCl3): δ (ppm) 7.83 (d, 2H, J = 4.8 Hz), 7.29 (d, 2H, J = 4.8 Hz). 13C{1H} NMR (100 MHz, CDCl3): δ (ppm) 174.0, 142.6, 138.6, 135.2, 125.0. FTIR (KBr, cm−1): 1646 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, s).
O, s).
General procedure for the synthesis of 3a and 3b
A mixture of benzaldehyde (2.4 mmol), benzo[1,2-b:4,3-b′]dithiophene-4,5-quinone (2.2 mmol), ammonium acetate (72.6 mmol) and acetic acid (20 mL) was heated to 100 °C overnight. The green solution was cooled to room temperature, and 15 mL water was added to stir for 15 min at room temperature. The solution was filtered in a Buchner funnel. The product was washed thoroughly in water and hexane, dried and taken to the next step.General procedure for the synthesis of 4a and 4b
To an oven dried Schlenk flask, the previously prepared compounds (3a/3b) (0.8 mmol) in DMF (30 mL) were taken, K2CO3 (2.1 mmol) was added and heated to 95 °C for 3 h and then cooled to room temperature. To it 1-iodoheptane (0.9 mmol) was added slowly. The reaction mixture was heated to 95 °C overnight. After cooling to room temperature the reaction mixture was poured in 10 mL water. The organic phase was extracted by ethyl acetate via repeated washing in water and dried over anhydrous MgSO4. The solvent was removed under rotary evaporation. The crude product was purified by silica gel column chromatography (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 10
hexane = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) to give a solid product (4a/4b) (50–60% yield).
90) to give a solid product (4a/4b) (50–60% yield).
FTIR (KBr, cm−1): 473 (νCu–N w), 1458 (νN–O, asymmetric, b), 1261 (νN–O, symmetric, b). MALDI-TOF: C48H46Br2N4S4Cu, calculated value 1029.520, experimental 1029.700 ([(4b)2·Cu(II)]+ molecular ion peak).
General procedure for the synthesis of 5a and 5b
A solution of 4a/4b (1.7 mmol) in THF (10 mL) was taken in a 100 mL oven dried Schlenk RB flask. N-Bromosuccinamide (3.8 mmol) was added portion-wise in 10 min intervals with continuous stirring at room temperature. Completion of the reaction was monitored by TLC. After completion of the reaction the solvent was then removed by evaporation under reduced pressure and the crude product was purified by silica gel column chromatography (hexane as the eluent) to give a brown solid of 5a/5b (85–90% yield).Synthesis of polymers
Then the solvent was concentrated to a minimum volume (2 mL) and the polymer was precipitated to stirring hexane. After complete precipitation hexane was decanted and the polymer was washed another two times with hexane. The polymer was further purified through Soxhlet extraction using hexane and methanol. Finally the polymer was isolated from DCM extraction. The DCM part was evaporated to afford the brown coloured polymer (0.3 g, 68% yield). 1H NMR (600 MHz, CDCl3): δ (ppm) 7.87–7.61 (m), 4.46–4.34 (m, 2H), 1.92–1.84 (m, 2H), 1.27–1.18 (m, 8H), 0.85–0.82 (m, 3H). 13C{1H} NMR (150 MHz, CDCl3): δ (ppm) 152.3, 139.8, 132.2, 132.0, 131.4, 130.2, 127.7, 124.5, 123.2, 122.6, 120.0, 46.2, 31.9, 31.3, 28.9, 26.3, 22.7, 14.0. λmax (ε, L mol−1 cm−1): 400 nm (1.71 × 104), 279 nm (2.70 × 104). Tetradetector GPC data: Mn = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519, Mw = 25
519, Mw = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953, PDI = 1.67.
953, PDI = 1.67.
P1 was synthesized following a similar protocol as described for P2, starting from 5a (0.05 g, 0.08 mmol) (yield 0.036 g, 65%). 1H NMR (600 MHz, CDCl3): δ (ppm) 7.66–7.33 (m), 4.51–4.46 (m, 3H), 2.46 (s, 3H), 1.90–1.84 (m, 2H), 1.26–1.15 (m, 8H), 0.81–0.79 (m, 3H). λmax (ε, L mol−1 cm−1): 395 nm (2.26 × 104), 279 nm (2.73 × 104). Tetradetector GPC data: Mn = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 799, Mw = 26
799, Mw = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781, PDI = 1.81.
781, PDI = 1.81.
X-ray data collection and refinement
The yellow coloured needle-like single crystals of 4b suitable for X-ray crystallography were obtained by layering a THF solution of 4b on water. Single-crystal X-ray structural studies were performed on a Bruker-APEX-II CCD X-ray diffractometer equipped with an Oxford Instruments low temperature attachment. Data were collected at 100(2) K using graphite-monochromated Mo Kα radiation (λα = R 0.71, 0 73 Å). The frames were indexed, integrated, and scaled using the SMART and SAINT software package,26 and the data were corrected for absorption using the SADABS program.27 Pertinent crystallographic data for 4b are summarized in Table S1.† Two independent molecules of 4b were located in the asymmetric unit with negligible differences in their metrical parameters. CCDC-1412125 contains the supplementary crystallographic data for this paper.† The structure was solved and refined using the SHELX suite of programs.28 All molecular structures were generated using ORTEP-3 for Windows Version 2.02.29 The hydrogen atoms were included in the geometrically calculated positions in the final stages of the refinement and were refined according to the typical riding model. All non-hydrogen atoms were refined with anisotropic thermal parameters.Acknowledgements
Authors acknowledge the financial support from the DST-SERB, India through Fast Track scheme (SB/FT/CS-010/2012). Authors thank Prof. Nilmoni Sarkar’s group (Dept. of Chemistry, IIT Kharagpur) for providing TCSPC measurement facilities. SKP thanks IIT Kharagpur for funding the purchase of the electrochemical workstation and Multidetector GPC through SGIRG (IIT/SRIC/CHY/PBR/2014-15/44) and SGDRI (IIT/SRIC/CHY/NPA/2014-15/81) grants (Competitive Research Infrastructure Seed Grant) respectively. A doctoral fellowship from IIT Kharagpur (to D. G.) is gratefully acknowledged.References
- (a) M. Boissinot, H. A. Ho and M. Leclerc, Angew. Chem., Int. Ed., 2002, 41, 1548 CrossRef; (b) F. Feng, F. He, L. An, S. Wang, Y. Li and D. Zhu, Adv. Mater., 2008, 20, 2959 CrossRef CAS PubMed; (c) G. Harsanyi, Polymer Films in Sensor Application, Taylor & Francis Group, Pennsylvania, USA, 1995 Search PubMed; (d) Y. Bao, H. Wang, Q. Li, B. Liu, W. Bai, B. Jin and R. Bai, Macromolecules, 2012, 45, 3394 CrossRef CAS; (e) A. Rostami and M. S. Taylor, Macromol. Rapid Commun., 2012, 33, 21 CrossRef CAS PubMed; (f) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- For recent reviews and book, see (a) H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210 RSC; (b) H. Zhu, J. Fan, B. Wang and X. Peng, Chem. Soc. Rev., 2015, 44, 4337 RSC; (c) R. B. Thompson, Fluorescence sensors and biosensors, Taylor & Francis Group, New York, 2006 Search PubMed; (d) L. J. Fan, Y. Zhang, C. B. Murphy, S. E. Angell, M. F. L. Parker, B. R. Flynn and W. E. Jones Jr, Coord. Chem. Rev., 2009, 253, 410 CrossRef CAS PubMed; (e) X. Feng, L. Liu, S. Wang and D. Zhu, Chem. Soc. Rev., 2010, 39, 2411 RSC.
- (a) M. Bouachrine, J. P. Lere-Porte, J. J. E. Moreau, F. Sereinspirau and C. Torreilles, J. Mater. Chem., 2000, 10, 263 RSC; (b) J. Pei, X. L. Liu, W. L. Yu, Y. H. Lai, Y. H. Niu and Y. Cao, Macromolecules, 2002, 35, 7274 CrossRef CAS; (c) T. Yasuda, I. Yamaguchi and T. Yamamoto, Adv. Mater., 2003, 15, 293 CrossRef CAS PubMed; (d) M. Kimura, T. Horai, K. Hanabusa and H. Shirai, Adv. Mater., 1998, 10, 459 CrossRef CAS; (e) Y. Eichen, G. Nakhmanovich, V. Gorelik, O. Epshtein, J. M. Poplawski and E. Ehrenfreund, J. Am. Chem. Soc., 1998, 120, 10463 CrossRef CAS; (f) M. Zhang, P. Lu, Y. G. Ma and J. C. Shen, J. Phys. Chem. B, 2003, 107, 6535 CrossRef CAS; (g) C. B. Murphy, Y. Zhang, T. Troxler, V. Ferry, J. J. Martin and W. E. Jones, J. Phys. Chem. B, 2004, 108, 1537 CrossRef CAS; (h) C. Xing, Z. Shi, M. Yu and S. Wang, Polymer, 2008, 49, 2698 CrossRef CAS PubMed; (i) C. Li, C. Zhou, H. Zheng, X. Yin, Z. Zuo, H. Liu and Y. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1998 CrossRef CAS PubMed; (j) T. M. Swager, Acc. Chem. Res., 1998, 31, 201 CrossRef CAS.
- (a) F. Tisato, C. Marzano, M. Porchia, M. Pellei and C. Santini, Med. Res. Rev., 2010, 30, 708 CAS; (b) M. C. Linder, Biochemistry of copper, Plenum Press, New York, 1991 Search PubMed; (c) V. Desai and S. G. Kaler, Am. J. Clin. Nutr., 2008, 88, 8558 Search PubMed; (d) K. G. Daniel, R. H. Harbach, W. C. Guida and Q. P. Dou, Front. Biosci., 2004, 9, 2652 CrossRef CAS PubMed; (e) P. C. Bull, G. R. Thomas, M. J. Rommens, J. R. Forbes and W. D. Cox, Nat. Genet., 1993, 5, 327 CrossRef CAS PubMed; (f) B. E. Kim, T. Nevitt and D. Thiele, Nat. Chem. Biol., 2008, 4, 176 CrossRef CAS PubMed.
- (a) K. J. Barnham, C. L. Masters and I. A. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205 CrossRef CAS PubMed; (b) R. R. Crichton, D. T. Dexter and J. R. Ward, Coord. Chem. Rev., 2008, 252, 1189 CrossRef CAS PubMed.
- (a) C. Vulpe, B. Levinson, S. Whitney, S. Packman and J. Gitschier, Nat. Genet., 1993, 3, 7 CrossRef CAS PubMed; (b) S. Lutsenko, A. Gupta, L. J. Burkhead and V. Zuzel, Arch. Biochem. Biophys., 2008, 476, 22 CrossRef CAS PubMed.
- (a) J. J. Pinto, C. Moreno and M. V. Garcia, Talanta, 2004, 64, 562 CrossRef CAS PubMed; (b) N. Pourreza and R. Hoveizavi, Anal. Chim. Acta, 2005, 549, 124 CrossRef CAS PubMed; (c) A. P. S. Gonzales, M. A. Firmino, C. S. Nomura, F. R. P. Rocha, P. V. Oliveira and I. Gaubeur, Anal. Chim. Acta, 2009, 636, 198 CrossRef CAS PubMed; (d) D. Y. Fu and D. Yuan, Spectrochim. Acta, Part A, 2007, 66, 434 CrossRef PubMed; (e) A. A. Ensafi, T. Khayamian, A. Benvidi and E. Mirmomtaz, Anal. Chim. Acta, 2006, 561, 225 CrossRef CAS PubMed; (f) Y. Fang, Y. Zhou, Q. Rui and C. Yao, Organometallics, 2015, 34, 2962 CrossRef CAS.
- (a) A. P. Demchenko, Introduction to Fluorescence Sensing, Spinger, USA, 2009 Search PubMed; (b) C. Zhang, S. Pu, Z. Sun, C. Fan and G. Liu, J. Phys. Chem. B, 2015, 119, 4673 CrossRef CAS PubMed; (c) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed.
- Representative references regarding fluorescence probe for heavy metal ions (Zn2+, Pb2+, Hg2+, Cd2+ and others); see (a) R. Zhou, G. Gao, J. Lan and J. You, Chem. Commun., 2011, 47, 6668 RSC; (b) M. Alfonso, A. Tarraga and P. Molina, Dalton Trans., 2010, 39, 8637 RSC; (c) M. Alfonso, A. Tarraga, A. Espinosa and P. Molina, Org. Lett., 2011, 13, 2078 CrossRef CAS PubMed; (d) M. Alfonso, A. Tarraga and P. Molina, J. Org. Chem., 2011, 76, 939 CrossRef CAS PubMed; (e) W. Hong, W. Li, X. Hu, B. Zhao and D. Zhang, J. Mater. Chem., 2011, 21, 17193 RSC; (f) L. N. Neupane, J. M. Kim and C. R. Lohani, J. Mater. Chem., 2012, 22, 4003 RSC; (g) J. F. Zhang, S. Kim, J. H. Han, S. J. Lee, Q. Y. Cao, S. J. Lee, T. Pradhan, J. S. Kim and C. Kang, Org. Lett., 2011, 13, 5294 CrossRef CAS PubMed; (h) Y. Pang and Y. Xu, Dalton Trans., 2011, 40, 1503 RSC; (i) Y. Pang and Y. Xu, Chem. Commun., 2010, 46, 4070 RSC; (j) K. Hanaoka, Y. Muramatsu and T. Nagano, Chem.–Eur. J., 2010, 16, 568 CrossRef CAS PubMed; (k) M. Taki, M. Desaki, Y. Yamamoto, A. Ojida, S. Iyoshi, I. Hamachi, Y. Yamamoto and T. Hirayama, J. Am. Chem. Soc., 2008, 130, 12564 CrossRef CAS PubMed; (l) T. Cheng, Y. Xu, S. Zhang, L. Duan and X. Qian, J. Am. Chem. Soc., 2008, 130, 16160 CrossRef CAS PubMed; (m) C. Lu, Z. Xu, J. Cui, R. Zhang and X. Qian, J. Org. Chem. Soc., 2007, 72, 3554 CrossRef CAS PubMedFor selective sensing of Cu2+; see (n) S. H. Jung, S. P. Kwon, W. J. Lee, I. J. Kim, S. C. Hong, W. J. Kim, S. Yan, Y. J. Lee, H. J. Lee, T. Joo and S. J. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef PubMed; (o) P. Kaur, S. Kaur and S. Singh, Org. Biomol. Chem., 2012, 10, 1497 RSC; (p) S. Sarkar and R. Shunmugam, ACS Appl. Mater. Interfaces, 2013, 5, 7379 CrossRef CAS PubMed; (q) W. Wang, Q. Wen, Y. Zhang, X. Fei, Y. Li, Q. Yang and X. Xu, Dalton Trans., 2013, 42, 1827 RSC; (r) M. Shellaiah, Y. C. Rajan and H. C. Lin, J. Mater. Chem., 2012, 22, 8976 RSC; (s) M. Shellaiah, Y. H. Wu, A. Singh, M. V. R. Raju and H. C. Lin, J. Mater. Chem. A, 2013, 1, 1310 RSC; (t) Y. Chen, C. Zhu, J. Cen, J. Li, W. He, Y. Jiao and Z. Guo, Chem. Commun., 2013, 49, 7632 RSC; (u) D. Guo, Z. Dong, C. Luo, W. Zan, S. Yan and X. Yao, RSC Adv., 2014, 4, 5718 RSC; (v) R. Sheng, P. Wang, Y. Gao, Y. Wu, W. Liu, J. Ma, H. Li and S. Wu, Org. Lett., 2008, 10, 5015 CrossRef CAS PubMed; (w) S. Goswami, D. Sen and N. K. Das, Org. Lett., 2010, 12, 856 CrossRef CAS PubMed.
- (a) S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef CAS PubMed; (b) J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 5321 CrossRef CAS; (c) J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS; (d) L. X. Chen, W. J. H. Jager, M. P. Niemczyk and M. R. Wasielewski, J. Phys. Chem. A, 1999, 103, 4341 CrossRef CAS; (e) X. Liu and J. Zhu, J. Phys. Chem. B, 2009, 113, 8214 CrossRef CAS PubMed; (f) X. Liu, X. Zhou, X. Shu and J. Zhu, Macromolecules, 2009, 42, 7634 CrossRef CAS; (g) Q. Zhou and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 7017 CrossRef CAS; (h) B. Wang and M. R. Wasielewski, J. Am. Chem. Soc., 1997, 119, 12 CrossRef CAS.
- (a) R. Satapathy, Y. H. Wu and H. C. Lin, Org. Lett., 2012, 14, 2564 CrossRef CAS PubMed; (b) R. Satapathy, Y. H. Wu and H. C. Lin, Chem. Commun., 2012, 48, 5668 RSC.
- (a) I. Welterlich and B. Tieke, Macromolecules, 2011, 44, 4194 CrossRef CAS; (b) C. Kaes, A. Katz and M. W. Hosseini, Chem. Rev, 2000, 100, 3553 CrossRef CAS PubMed; (c) T. Yasuda and T. Yamamoto, Macromolecules, 2003, 36, 7513 CrossRef CAS; (d) B. Liu, W. L. Yu, J. Pei, S. Y. Liu, Y. H. Lai and W. Huang, Macromolecules, 2001, 34, 7932 CrossRef CAS.
- I. Osaka and R. D. Mccullough, Acc. Chem. Res., 2008, 41, 1202 CrossRef CAS PubMed.
- (a) L. S. Miguel and A. J. Matzger, Macromolecules, 2007, 40, 9233 CrossRef; (b) E. Bundgaard and F. C. Krebs, Macromolecules, 2006, 39, 2823 CrossRef CAS.
- (a) Z. Bao, W. K. Chan and L. Yu, J. Am. Chem. Soc., 1995, 117, 12426 CrossRef CAS; (b) P. M. Beaujuge, W. Pisula, H. N. Tsao, S. Ellinger, K. Mullen and J. R. Reynolds, J. Am. Chem. Soc., 2009, 131, 7514 CrossRef CAS PubMed.
- (a) S. Sirilaksanapong, M. Sukwattanasinitt and P. Rashatasakhon, Chem. Commun., 2012, 48, 293 RSC; (b) J. Hatai, S. Pal and S. Bandyopadhyay, Tetrahedron Lett., 2012, 53, 4357 CrossRef CAS PubMed.
- (a) S. Cao, Z. Pei, Y. Xu, R. Zhang and Y. Pei, RSC Adv., 2015, 5, 45888 RSC; (b) J. R. Lakowicz, Principle of Fluorescence Spectroscopy, Plenum Publishing Corporation, New York, 2nd edn, 1991 Search PubMed.
- (a) L. Duan, Y. Xu and X. Qian, Chem. Commun., 2008, 44, 6339 RSC; (b) D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596 CrossRef CAS PubMed.
- (a) Z. Lin, G. Zhang, W. Yang, B. Qiu and G. Chen, Chem. Commun., 2012, 48, 9918 RSC; (b) C. Kaewtomg, J. Noiseephum, Y. Uppa, T. Tuntulani and B. Pulpoka, New J. Chem., 2010, 34, 1104 RSC; (c) E. A. Albers, S. V. Okreglak and J. C. Chang, J. Am. Chem. Soc., 2006, 128, 9640 CrossRef PubMed.
- (a) X. L. Tang, X. H. Peng, W. Dou, J. Mao, J. R. Zheng, W. W. Qin, W. S. Liu, J. Chang and X. J. Yao, Org. Lett., 2008, 10, 3653 CrossRef CAS PubMed; (b) K. M. K. Swamy, S. K. Ko, S. K. Kwon, H. N. Lee, C. Mao, J. M. Kim, K. H. Lee, J. Kim, I. Shin and J. Yoon, Chem. Commun., 2008, 5915 RSC.
- (a) H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed; (b) C. Xing, M. Yu, S. Wang, Z. Shi, Y. Li and D. Zhu, Macromol. Rapid Commun., 2007, 28, 241 CrossRef CAS PubMed.
- (a) G. D. Santis, L. Fabbrizzi, M. Licchelli, N. Sardona and A. H. Velders, Chem.–Eur. J., 1996, 2, 1243 CrossRef PubMed; (b) R. Bergonzi, L. Fabbrizzi, M. Licchelli and C. Mangano, Coord. Chem. Rev., 1998, 170, 31 CrossRef CAS.
- (a) Y. Fu, Q. C. Feng, X. Iang, H. Xu, M. Li and S. Q. Zang, Dalton Trans., 2014, 43, 5815 RSC; (b) M. Li, H. Ge, R. L. Arrowsmith, V. Mirabello, S. W. Botchway, W. Zhu, S. I. Pascu and T. D. James, Chem. Commun., 2014, 50, 11806 RSC.
- (a) M. Gullotti, L. Casella, A. Pintar and E. Suardi, J. Chem. Soc., Dalton Trans., 1989, 1, 1979 RSC; (b) G. Xu, J. Fan and K. Jiao, Electroanalysis, 2008, 20, 1209 CrossRef CAS PubMed.
- D. R. Coulson, L. C. Satek and S. O. Grim, Inorg. Synth., 1972, 13, 121 Search PubMed.
- SAINT+ Software for CCD Diffractometers, Bruker AXS, Madison, WI, 2000 Search PubMed.
- G. M. Sheldrick, SADABS Program for Correction of Area Detector Data, University of Göttingen, Göttingen, Germany, 1999 Search PubMed.
- (a) SHELXTL Package, version 6.10, Bruker AXS, Madison, WI, 2000 Search PubMed; (b) G. M. Sheldrick, SHELXS-86 and SHELXL-97, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and characterization data, full photophysical and sensing studies, and X-ray crystallographic data. CCDC 1412125. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14079j |
| This journal is © The Royal Society of Chemistry 2015 |