Arylhydrazones of barbituric acid: synthesis, coordination ability and catalytic activity of their CoII, CoII/III and CuII complexes toward peroxidative oxidation of alkanes†
Jessica Palmucciab,
Kamran T. Mahmudov*ac,
M. Fátima C. Guedes da Silva*a,
Luísa M. D. R. S. Martins *ad,
Fabio Marchettib,
Claudio Pettinarie and
Armando J. L. Pombeiro*a
*ad,
Fabio Marchettib,
Claudio Pettinarie and
Armando J. L. Pombeiro*a
aCentro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1049–001 Lisbon, Portugal. E-mail: kamran_chem@mail.ru; kamran_chem@yahoo.com; fatima.guedes@tecnico.ulisboa.pt; lmartins@deq.isel.ipl.pt; pombeiro@tecnico.ulisboa.pt
bSchool of Science and Technology, University of Camerino, Chemistry Section, via S. Agostino 1, 62032 Camerino, Italy
cDepartment of Chemistry, Baku State University, Z. Xalilov Str. 23, Az 1148 Baku, Azerbaijan
dChemical Engineering Department, Instituto Superior de Engenharia de Lisboa, Instituto Politécnico de Lisboa, R. Conselheiro Emídio Navarro, 1959-007 Lisboa, Portugal
eSchool of Pharmacy, University of Camerino, Chemistry Section, via S. Agostino 1, 62032 Camerino, Italy
First published on 29th September 2015
Abstract
New ortho-substituted arylhydrazones of barbituric acid, 5-(2-(2-hydroxyphenyl)hydrazono) pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and the sodium salt of 2-(2-(2,4,6-trioxotetra-hydropyrimidin-5(2H)-ylidene)hydrazinyl)benzenesulfonic acid (H4L2), [Na(H3L2)(μ-H2O)(H2O)2]2 (1), were used in the synthesis of CuII, CoII and CoII/III complexes, [Cu(H2L1)(H2O)(im)]·3H2O (im = imidazole) (2), [Co(H2O)6][Co(H2L1)2]2·8H2O (3), [Co(H2L2)(im)3] (4), [Cu(H2L2)(im)2]·H2O (5) and [Co(H2O)6][H3L2]2·8H2O (6). The complexes are water soluble and the mono- or di-deprotonated ligands display different coordination modes, depending on the synthetic conditions. The electrochemical behaviour of all the compounds was investigated by cyclic voltammetry and controlled potential electrolysis, revealing that the ligands are also redox active. All the compounds were evaluated as catalysts for the peroxidative (with H2O2) oxidation of cyclohexane at room temperature. The compounds 2 and 3 are the most active ones (yields up to 21% and TON up to 213 are achieved, in the presence of 3).
Introduction
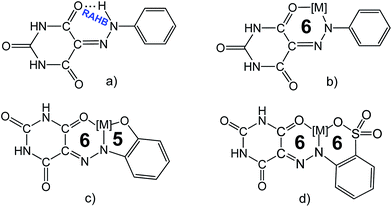
Barbiturates, besides their biological significance, can also be used as precursors in the synthesis of numerous compounds, such as polymers,1a pigments,1b dyes1c and vitamin B2,1d and as building blocks in the construction of supramolecular structures owing to both their H-bond donor and acceptor capabilities.2 The rich coordination and supramolecular chemistry of barbiturates has been reviewed recently by some of us,2 but data on the complexes with arylhydrazones of barbituric acid (AHBAs, Scheme 1a) ligands are rather scarce2,3 and mainly involve copper and zinc complexes.2,3g,h All the reported AHBA ligands are stabilized in the hydrazone form with the formation of intramolecular resonance-assisted hydrogen bonding (RAHB)2 between the hydrazone![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH– moiety and a carbonyl group giving a six-membered cycle (Scheme 1a). In order to increase the coordination sites of AHBA ligands, we decided to focus on functionalized forms, in particular the ortho-SO3H and –OH substituted versions (Scheme 1c–d), in view of their promising coordination features. Metallacycles can be obtained and, as shown in related compounds,4 the ortho-SO3H group can give more stable two six-membered metallacycles than the matallacycles associated to the ortho-OH and to the unsubstituted AHBA ligands (Scheme 1b–d). Moreover, functionalization of a ligand with an hydrophilic polar group, such as –OH, –SO3H, is a common way to increase the water solubility of its complexes, which is important for catalysis in aqueous medium.4,5
N–NH– moiety and a carbonyl group giving a six-membered cycle (Scheme 1a). In order to increase the coordination sites of AHBA ligands, we decided to focus on functionalized forms, in particular the ortho-SO3H and –OH substituted versions (Scheme 1c–d), in view of their promising coordination features. Metallacycles can be obtained and, as shown in related compounds,4 the ortho-SO3H group can give more stable two six-membered metallacycles than the matallacycles associated to the ortho-OH and to the unsubstituted AHBA ligands (Scheme 1b–d). Moreover, functionalization of a ligand with an hydrophilic polar group, such as –OH, –SO3H, is a common way to increase the water solubility of its complexes, which is important for catalysis in aqueous medium.4,5
From other perspective, due to proton-donor or -acceptor sites of imidazole (im), it has been used as an auxiliary ligand or deprotonating agent in the synthesis and design of water soluble coordination compounds.4 Thus, the use of im as an auxiliary ligand or a deprotonating agent for the weakening/destroying RAHB system in the complexation of AHBA ligands with transition metals such as CuII, CoII, etc. is of practical importance for the development of new synthetic methods.
No application in catalysis of any metal complex of AHBA had been reported so far. Thus, the preparation and characterization of the first CoII and CoII/III complexes, as well as of CuII, are of significant interest due to, e.g., their possible catalytic applications in the peroxidative oxidation of alkanes, for instance cyclohexane. Thus, another main aim of the current work concerns the extension of the application of AHBA complexes to this field, i.e. oxidative catalysis. The partial oxidation of cyclohexane is the main industrial route to cyclohexanone and cyclohexanol, which are important intermediates in the production of adipic acid and caprolactam, also used in the manufacture of nylon-6 and nylon-66 polymers.6 However, the industrial aerobic oxidation of cyclohexane to the alcohol/ketone mixture, catalysed by Co salts, requires the use of considerable temperature (150–170 °C) and proceeds with a low yield (∼4%) in order to achieve a selectivity of ca. 85%.6h,k Hence, it is of a great practical interest to develop a more efficient, as well as easily synthesizable catalyst for the selective cyclohexane oxidation process able to operate under milder conditions. In addition, such catalyst should be active in a reaction medium that employs either hydrogen peroxide or preferably dioxygen to become an attractive alternative to the currently used industrial processes.
Thus, in this work we focused on the following aims: (i) synthesis of new water soluble AHBA ligands bearing ortho -OH and -SO3H groups, namely 5-(2-(2-hydroxyphenyl)hydrazono) pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and sodium salt of 2-(2-(2,4,6-trioxotetrahydro-pyrimidin-5(2H)-ylidene)hydrazinyl)benzenesulfonic acid (H4L2), and their application in the preparation of new water soluble CoII, CoII/III and CuII-AHBA complexes; (ii) study of the physicochemical and electrochemical properties of the ligands and complexes; (iii) application of the complexes as catalyst precursors for the peroxidative oxidation of cyclohexane in aqueous acetonitrile medium, under mild conditions.
Results and discussion
Synthesis and characterization of H4L1, NaI salt and CoII, CoII/III and CuII complexes
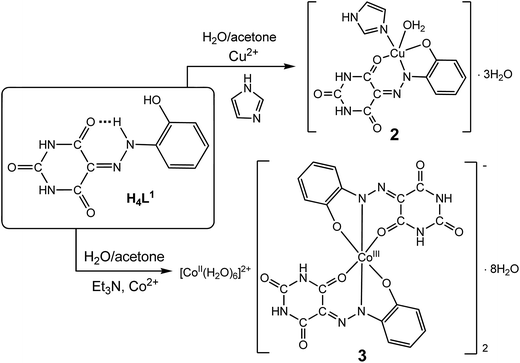
The new AHBAs, namely 5-(2-(2-hydroxyphenyl)hydrazono)pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and sodium salt of 2-(2-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)hydrazinyl) benzenesulfonic acid (H4L2), [Na(H3L2)(μ-H2O)(H2O)2]2 (1), were prepared by a modified known7 aqueous diazotization of 2-aminophenol and 2-aminobenzenesulfonic acid and subsequent coupling with barbituric acid in basic medium, respectively (Scheme 2). H4L1 and 1 are highly soluble in DMSO, DMF, water, methanol, acetone and acetonitrile. Both compounds are stabilized in DMSO-d6 solution in the hydrazone form, as reported analogues.2,7d In fact, 1H-NMR spectra of H4L1 and 1 in DMSO-d6 solution at room temperature show only one signal at δ 14.44 and 14.80, respectively, assigned to the proton of the protonated nitrogen atom adjacent to the aryl unit (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH–hydrazone form). In accord, two peaks of the carbonyl groups in the 13C NMR spectra (see Experimental) indicate that one of these groups undergoes a shift due to hydrogen bonding of the carbonyl moiety with the hydrazone NH group. This is in agreement with the low field chemical shift (14.44 and 14.80 ppm) of the hydrazone NH proton and is also consistent with IR data: the stretching bands ν(C
N–NH–hydrazone form). In accord, two peaks of the carbonyl groups in the 13C NMR spectra (see Experimental) indicate that one of these groups undergoes a shift due to hydrogen bonding of the carbonyl moiety with the hydrazone NH group. This is in agreement with the low field chemical shift (14.44 and 14.80 ppm) of the hydrazone NH proton and is also consistent with IR data: the stretching bands ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(C
O) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H) are at 1697 and 1643 (for H4L1), 1677 and 1604 (for 1) cm−1, respectively, the latter being shifted on account of the H-bond.2,7d Elemental analysis and the ESI-MS peaks at 249.2 [Mr + H]+ and 702.1 [Mr − 4H2O + H]+ support the formulation of H4L1 and 1, respectively (Fig. 1S and 2S†). Moreover, X-ray diffraction analysis of 1 is demonstrate that it is stabilized in the hydrazone form (see below).
O⋯H) are at 1697 and 1643 (for H4L1), 1677 and 1604 (for 1) cm−1, respectively, the latter being shifted on account of the H-bond.2,7d Elemental analysis and the ESI-MS peaks at 249.2 [Mr + H]+ and 702.1 [Mr − 4H2O + H]+ support the formulation of H4L1 and 1, respectively (Fig. 1S and 2S†). Moreover, X-ray diffraction analysis of 1 is demonstrate that it is stabilized in the hydrazone form (see below).
Reaction of H4L1 with CuII or CoII (nitrate salts) in the presence of imidazole (im) and triethylamine (Et3N) in acetone–water mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) lead to [Cu(H2L1)(H2O)(im)]·3H2O (2) and [Co(H2O)6][Co(H2L1)2]2·8H2O (3), respectively (Scheme 2, see Experimental for details). The added base (im or Et3N) conceivably weakens the hydrogen-bonded system in H4L1 and its reactivity increases towards N–H deprotonation and coordination to the metal ions. Both complexes are characterized by elemental analysis, ESI-MS spectra, IR spectroscopy and single crystal X-ray diffraction (see below). In the IR spectra of 2 and 3, the ν(C
1, v/v) lead to [Cu(H2L1)(H2O)(im)]·3H2O (2) and [Co(H2O)6][Co(H2L1)2]2·8H2O (3), respectively (Scheme 2, see Experimental for details). The added base (im or Et3N) conceivably weakens the hydrogen-bonded system in H4L1 and its reactivity increases towards N–H deprotonation and coordination to the metal ions. Both complexes are characterized by elemental analysis, ESI-MS spectra, IR spectroscopy and single crystal X-ray diffraction (see below). In the IR spectra of 2 and 3, the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(C
O) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) signals appear at 1714 and 1586, 1719 and 1594 cm−1, respectively, values that are significantly shifted in relation to the corresponding signals of H4L1 (1731 and 1697 ν(C
N) signals appear at 1714 and 1586, 1719 and 1594 cm−1, respectively, values that are significantly shifted in relation to the corresponding signals of H4L1 (1731 and 1697 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 ν(C
O), 1597 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)). Mass spectrometry of 2 and 3 dissolved in methanol shows parent peaks at m/z = 310.7 [C10H6CuN4O4 + H]+ (for 2) and 167.0 [Co(H2O)6]2+, 551.2 [C20H12CoN8O8]− (for 3) (Fig. 3S and 4S†). Elemental analyses are also in agreement with the proposed formulations, which are also supported by X-ray crystallography.
N)). Mass spectrometry of 2 and 3 dissolved in methanol shows parent peaks at m/z = 310.7 [C10H6CuN4O4 + H]+ (for 2) and 167.0 [Co(H2O)6]2+, 551.2 [C20H12CoN8O8]− (for 3) (Fig. 3S and 4S†). Elemental analyses are also in agreement with the proposed formulations, which are also supported by X-ray crystallography.
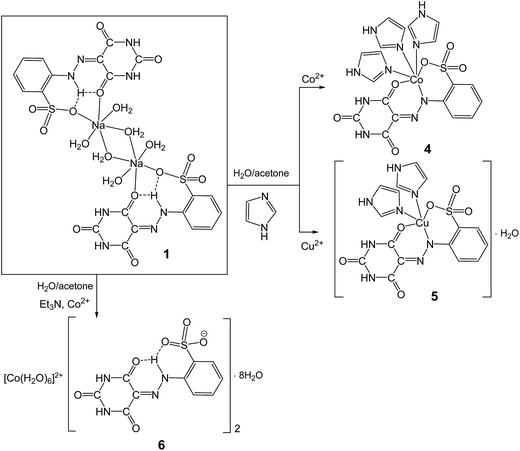
Reactions of Co(NO3)2·6H2O and Cu(NO3)2·2.5H2O with 1 in water–acetone mixture (1/3, v/v), in the presence of im led to the CoII and CuII complexes [Co(H2L2)(im)3] (4) and [Cu(H2L2)(im)2]·H2O (5), respectively (Scheme 3). The CoII salt of AHBA, [Co(H2O)6][H3L2]2·8H2O (6), was obtained by mixing (stirring for 10 min) of an acetone–water mixture (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution of 1, Et3N and Co(NO3)2·6H2O at 80 °C (Scheme 3).
1, v/v) solution of 1, Et3N and Co(NO3)2·6H2O at 80 °C (Scheme 3).
All the isolated compounds, 4–6, were characterized by elemental analysis, ESI-MS, IR spectroscopy and single crystal X-ray diffraction. In the IR spectra of 4, 5 and 6, the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) signal appears at 1664, 1633 and 1675 cm−1, respectively, values that are significantly shifted in relation to the corresponding signal of 1 (1677 cm−1). The ESI mass spectra of 4, 5 and 6 dissolved in CH3OH show the peaks at m/z = 574.0 [{Co(im)3H2L2} + H]+, 510.0 [{[Cu(im)2H2L2]·H2O} − H2O + H]+, and 682.1 [Co(H3L2)2 + H]+ (Fig. 5S–7S†). Elemental analyses are consistent with the proposed formulations which are also supported by X-ray crystallography (see below). All the obtained complexes are soluble in water, methanol, DMF and DMSO, but later one is the best solvent for them.
O) signal appears at 1664, 1633 and 1675 cm−1, respectively, values that are significantly shifted in relation to the corresponding signal of 1 (1677 cm−1). The ESI mass spectra of 4, 5 and 6 dissolved in CH3OH show the peaks at m/z = 574.0 [{Co(im)3H2L2} + H]+, 510.0 [{[Cu(im)2H2L2]·H2O} − H2O + H]+, and 682.1 [Co(H3L2)2 + H]+ (Fig. 5S–7S†). Elemental analyses are consistent with the proposed formulations which are also supported by X-ray crystallography (see below). All the obtained complexes are soluble in water, methanol, DMF and DMSO, but later one is the best solvent for them.
The wavelength (λmax) and molar absorption values (ε) of all compounds in the UV-vis region 200–700 nm have been recorded in water solution (Table 1S and Fig. 8S†). In the spectra of H4L1 and 1–6 the bands at 234–270 nm are due to π–π* electronic transition, while the bands at 352–482 nm are mainly of n–π* type.7b
X-ray diffraction analyses
Crystals of 1–6 suitable for X-ray diffraction analysis were obtained upon crystallization from water–acetone mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) (the schematic representations of their formulae are shown in Schemes 2 and 3). The structure of 1 (Fig. 9S, ESI†) will not be discussed since it is a preliminary one (78% completeness) and no further suitable crystals of the compound were obtained. Representative plots of 2–6 are depicted in Fig. 1. Crystallographic data and refinement parameters are given in Tables 2S and 3S† H-contacts for 2–6 are outlined in Table 4S† and depicted in Fig. 10S†).
4, v/v) (the schematic representations of their formulae are shown in Schemes 2 and 3). The structure of 1 (Fig. 9S, ESI†) will not be discussed since it is a preliminary one (78% completeness) and no further suitable crystals of the compound were obtained. Representative plots of 2–6 are depicted in Fig. 1. Crystallographic data and refinement parameters are given in Tables 2S and 3S† H-contacts for 2–6 are outlined in Table 4S† and depicted in Fig. 10S†).
In the mononuclear structures 2–5 an N-hydrazone atom, a phenoxyl (in 2 and 3) or a sulfonyl (in 4 and 5) O-atom and an additional O-atom from the pyrimidine trione moiety of the Ln ligands are involved in the chelation. The Ln ligands are almost planar in the structure of 2 as measured by the angle of 2.01° (2) between planes H and P (see above; Table 4S†). However, they are considerably twisted in 4 and 5 with angles of 34.49 and 38.46°, respectively, involving those planes; in 3 that parameter assumes intermediate values (8.61 and 11.42°).
In all the structures the bond distances of 1.202(8)–1.308(4) Å for the N–N hydrazone groups suggest a double bond character.
The structure of 3 comprises two CoIII (anionic part {[C20H12CoIIIN8O8]−}2) and one CoII (cationic part [Co(H2O)6]2+) complex units, one of them involving two ONO chelating hydrazone (H2L1)2− anions and the other six coordinated water molecules (Scheme 2). For the anionic part of 3, [Co(H2O)6]2+ is counter ion rendering the compound neutral. Both metal centres show slightly distorted octahedral environments as expressed by the octahedral quadratic elongation (OQE) values of 1.002 and 1.001, and octahedral angle variations (OAV) of 6.03 and 1.47°2, respectively.8b The hydrazone ligands in 3 are oriented in such a way that the two pairs of M–O bonds are mutually trans, as well as the pair of M–N bonds.
The CoII coordination sphere in 4 comprises one (H2L2)2− anion and three imidazole ligands accomplishing a distorted octahedral environment around the cobalt cation (OQE and OAV of 1.008 and 25.55°2, in this order). The Co–O bond lengths comprising the hydrazone derivative assume values of 2.104(5) and 2.195(4) Å and concern the Oketone and Osulfonyl, respectively; the Co–N distance, in turn, take a value of 2.118(5) Å. The Co–N distances concerning the imidazole ligands in 4 range from 2.110(5) to 2.134(6) Å, the longer one conceivably resulting from a trans effect of the coordinated sulfonyl O atom of the hydrazone ligand. The molecules of 4 are associated into infinite 1D chains along the crystallographic b axis by means of intermolecular N–H⋯O interactions involving one of the imidazole groups and one of the non-coordinated sulfonyl O-atoms (Fig. 10S and Table 4S†). The intermolecular metal⋯metal distance in 4 (8.608 Å) is considerably longer than that found in 3 (5.653 Å).
The CuII cation in 5 presents a square pyramidal geometry (τ5 = 0.02),8 the equatorial plane being occupied by the Oketone- and N-atoms from (H2L2)2−, as well as two N-atoms from two imidazole ligands; the axial site is engaged with one of the Osulfonyl atoms from the hydrazone. As found in 2, the coordination distance to the axial atom is longer than those to the equatorial atoms, although the tetragonal elongation8a is shorter (avg. 16%). The intermolecular metal⋯metal distance in 5 (6.603 Å) is slightly longer to that found in 2 (5.590 Å).
The structure of 6 also contains three-centred RAHB interactions4,7d in the anionic hydrazone units involving the Oketone- and Osulfonyl-atoms, which relates to the synergistic mutual reinforcement of intramolecular hydrogen bonding due to π-electron delocalization. The typical double bond lengths of Cpyrimidine–Nhydrazone in the structure of 6 [1.314(3) Å] and the formation of the intramolecular N–H⋯O RAHB contacts indicate that hydrazone is the preferred tautomeric form in these cases.
The structures of this work contain trapped water molecules (except 4) which interact among themselves and with the metal–organic (2, 3 and 5) hosts via intermolecular hydrogen bonds. Such interactions, together with others involving the O- and N-atoms of pyrimidine trione rings as well as the coordinated water molecules (in 2, 3 and 6; Fig. 10S†), give rise to intricate 3D supramolecular frameworks.
Electrochemical behaviour of H4L1 and 1–6
In view of the application of 2–6 as homogeneous catalysts in oxidation processes in acetonitrile solution, the redox properties of H4L1 and 1–6 were studied by cyclic voltammetry (CV) and controlled potential electrolysis (CPE) at a platinum working electrode at 25 °C in a 0.2 M [nBu4N][BF4]/NCMe solution. H4L1 and the NaI complex 1 revealed to be redox active (Table 1, Fig. 11S and 12S†), exhibiting, by CV, an irreversible oxidation wave at IEoxp 1.2 V vs. SCE and an irreversible reduction wave (IEredp) at ca. −0.9 to −1.1 V vs. SCE. Whereas the oxidation process appears not be affected by the nature or position of the substituents of the aromatic ring, the reduction one is more sensitive (a difference of 0.16 V vs. SCE is observed, Table 1). The redox processes at 1 are centred on the ligands. The copperII complexes 2 and 5 exhibit, besides the redox processes centred on the ligands (IEoxp and IEredp, Table 1), a two-electrons (as measured by CPE) irreversible cathodic wave at IIEredp −1.56 V or −1.67 vs. SCE, respectively, assigned to the CuII → Cu0 reduction (Fig. 13S†).9a,b The CPE performed at these potentials lead to the deposition of metallic copper which is clearly detected by its sharp oxidation (with desorption) wave at Eoxp = −0.09 (2) (Fig. 13S†) or −0.11 (5) V vs. SCE. For the Co complexes 3, 4 and 6, the irreversible oxidation waves (IEoxp and IIEoxp, Table 1) are conceivably centred on the organic ligands, as well as the irreversible reduction waves in the range of −0.8 to −1.0 V vs. SCE. However, in 3, the extra reversible reduction wave at IEred1/2 = −0.21 V is assigned to the CoIII → CoII reduction of the [CoIII(H2L1)2]− anionic complex part (see Fig. 14S†). Those potentials are much lower than that (IEoxp = 1.63 V, Table 1) observed for the CoII → CoIII oxidation in the salt Co(NO3)2 salt and also in mononuclear Co complexes bearing C-scorpionate9c or pyrazole9d ligands (1.03 to 1.52 V vs. SCE).9d| Compound | IEoxp | IIEoxp | IEredp (IE1/2) | IIEredp |
|---|---|---|---|---|
| a Potential values in volt ± 0.02 vs. SCE, in a 0.2 M [Bu4N][BF4]/NCMe solution, at a Pt disc working electrode determined by using the [Fe(η5-C5H5)2]0/+ redox couple (Eox1/2 = 0.42 V vs. SCE10) as internal standard at a scan rate of 200 mV s−1.b An anodic (with desorption) wave at Eoxp = −0.09 V is generated upon scan reversal following the second reduction wave.c An anodic (with desorption) wave at Eoxp = −0.11 V is generated upon scan reversal following the second reduction wave.d For comparison purposes. | ||||
| H4L1 | 1.22 | — | −0.93 | — |
| 1 | 1.21 | — | −1.09 | — |
| 2b | 0.78 | — | −0.82 | −1.56 |
| 3 | 0.92 | 1.08 | (−0.21) | −0.83 |
| 4 | 0.82 | 1.13 | −1.14 | — |
| 5c | 1.04 | — | −1.16 | −1.67 |
| 6 | 1.11 | 1.19 | −1.02 | — |
| Co(NO3)2·6H2Od | 1.63 | — | — | — |
Catalytic activity of metal complexes for cyclohexane oxidation
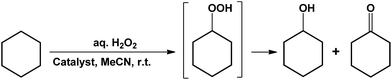
Compounds 1–6 and the proligand H4L1 were tested as catalysts for the peroxidative (with aqueous hydrogen peroxide) oxidation, at room temperature, of cyclohexane (CyH) to the mixture of cyclohexyl hydroperoxide (CyOOH, primary product) with cyclohexanol (CyOH) and cyclohexanone (Cy-H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) (final products) (Scheme 4). As expected, the non-metallic species H4L1, as well as the sodium compound 1, were not able to catalyse the above reaction in the tested conditions. The compounds 2–6 act as catalyst precursors for such an oxidation, 2 and 3 being the most active ones (Table 2). Thus, 2 and 3, derived from ortho-OH AHBA ligand, show high activity in comparison to other complexes from its ortho-SO3H substituted version. The catalytic activity of CoII complexes (4 and 6) is higher than of CuII one (5) derived from same ortho-SO3H substituted ligand, H4L2. Nevertheless, the catalytic performances of 4 and 6 are different (11 and 8% correspondingly, entries 5 and 9). Such differences possibly reflect the different coordination environment of CoII and labilities of the coordinated imidazole and water ligands in those complexes. The easy reduction of the CuII complex 2 detected by cyclic voltammetry (Table 1) can also have a favourable effect on its catalytic activity.
O) (final products) (Scheme 4). As expected, the non-metallic species H4L1, as well as the sodium compound 1, were not able to catalyse the above reaction in the tested conditions. The compounds 2–6 act as catalyst precursors for such an oxidation, 2 and 3 being the most active ones (Table 2). Thus, 2 and 3, derived from ortho-OH AHBA ligand, show high activity in comparison to other complexes from its ortho-SO3H substituted version. The catalytic activity of CoII complexes (4 and 6) is higher than of CuII one (5) derived from same ortho-SO3H substituted ligand, H4L2. Nevertheless, the catalytic performances of 4 and 6 are different (11 and 8% correspondingly, entries 5 and 9). Such differences possibly reflect the different coordination environment of CoII and labilities of the coordinated imidazole and water ligands in those complexes. The easy reduction of the CuII complex 2 detected by cyclic voltammetry (Table 1) can also have a favourable effect on its catalytic activity.
| Entry | Catalyst | n(Cat)/n(CyH) × 103 | n(H2O2)/n(Cat) × 10−3 | Yield (%)b CyOH Cy-H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O totale O totale |
TONc | CyOH/Cy-H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ratio O ratio |
Select.d (%) | ||
|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions (unless stated otherwise): MeCN (3 mL), cyclohexane (5.0 mmol), 0.5–20 μmol of 2–6, H2O2 (10.0 mmol), 6 h, r.t.; yield and TON determined by GC analysis (upon treatment with PPh3).b Molar yield (%) based on substrate, i.e. moles of products (cyclohexanol (CyOH) and cyclohexanone (Cy-H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)) per 100 mol of cyclohexane.c Turnover number (moles of product per mol of catalyst precursor).d Selectivity (moles of cyclohexanol and cyclohexanone per mole of converted substrate).e Moles of cyclohexanol + cyclohexanone per 100 moles of cyclohexane.f Reaction in the presence of HNO3 [n(HNO3)/n(catalyst) = 30].g Without addition of PPh3.h Reaction in the presence of CBrCl3 (5.0 mmol).i Reaction in the presence of Ph2NH (5.0 mmol).j Reaction performed under nitrogen atmosphere. O)) per 100 mol of cyclohexane.c Turnover number (moles of product per mol of catalyst precursor).d Selectivity (moles of cyclohexanol and cyclohexanone per mole of converted substrate).e Moles of cyclohexanol + cyclohexanone per 100 moles of cyclohexane.f Reaction in the presence of HNO3 [n(HNO3)/n(catalyst) = 30].g Without addition of PPh3.h Reaction in the presence of CBrCl3 (5.0 mmol).i Reaction in the presence of Ph2NH (5.0 mmol).j Reaction performed under nitrogen atmosphere. |
|||||||||
| 1 | 2 | 1 | 2 | 15.7 | 0.5 | 16.2 | 162 | 31.4 | 98 |
| 2f | 2 | 1 | 2 | 0.1 | 0.1 | 0.2 | 2 | 1.0 | 96 |
| 3 | 3 | 1 | 2 | 20.4 | 0.9 | 21.3 | 213 | 22.7 | 96 |
| 4f | 3 | 1 | 2 | 2.8 | 0.7 | 3.5 | 35 | 4.0 | 89 |
| 5 | 4 | 1 | 2 | 5.2 | 5.3 | 10.5 | 105 | 1.0 | 91 |
| 6f | 4 | 1 | 2 | 1.7 | 0.1 | 1.8 | 18 | 17.0 | 94 |
| 7 | 5 | 1 | 2 | 2.9 | 2.7 | 5.6 | 56 | 1.1 | 89 |
| 8f | 5 | 1 | 2 | 1.9 | 0.3 | 2.2 | 22 | 6.3 | 94 |
| 9 | 6 | 1 | 2 | 5.5 | 2.6 | 8.1 | 81 | 2.1 | 98 |
| 10f | 6 | 1 | 2 | 0.2 | 0 | 0.2 | 2 | — | 93 |
| 11g | 2 | 1 | 2 | 5.3 | 9.4 | 14.7 | 147 | 0.6 | 64 |
| 12 | 2 | 1 | 0.1 | 1.1 | 1.2 | 2.3 | 23 | 0.9 | 89 |
| 13 | 2 | 1 | 0.2 | 5.4 | 2.9 | 8.3 | 83 | 1.9 | 91 |
| 14 | 2 | 1 | 1 | 8.6 | 5.3 | 13.9 | 139 | 1.6 | 89 |
| 15 | 2 | 1 | 10 | 4.2 | 2.7 | 6.9 | 69 | 1.6 | 41 |
| 16 | 2 | 0.2 | 2 | 3.1 | 2.1 | 5.2 | 260 | 1.5 | 93 |
| 17 | 2 | 3 | 2 | 12.2 | 6.7 | 18.9 | 63 | 1.8 | 88 |
| 18 | 2 | 10 | 2 | 11.7 | 8.3 | 20.0 | 20 | 1.4 | 84 |
| 19 | 2 | 20 | 2 | 18.1 | 2.5 | 20.6 | 10 | 7.2 | 83 |
| 20h | 2 | 1 | 2 | 0.8 | 0.6 | 1.4 | 14 | 1.3 | 39 |
| 21i | 2 | 1 | 2 | 1.9 | 1.2 | 3.1 | 31 | 1.6 | 56 |
| 22j | 2 | 1 | 2 | 11.2 | 4.6 | 15.8 | 158 | 2.4 | 99 |
| 23 | Cu(NO3)2 | 1 | 2 | 2.9 | 1.2 | 4.1 | 41 | 2.4 | 39 |
| 24 | Co(NO3)2 | 1 | 2 | 4.7 | 2.6 | 7.3 | 73 | 1.8 | 61 |
| 25 | [Cu(im)4](NO3)2 | 1 | 2 | 4.3 | 3.3 | 7.6 | 76 | 1.3 | 73 |
| 26 | [Co(im)6](NO3)2 | 1 | 2 | 5.2 | 3.9 | 9.1 | 81 | 1.3 | 84 |
In fact, after 6 h stirring the reaction mixture (CyH, H2O2 30% aq. and the copper or cobalt catalyst 2 or 3, respectively, in MeCN) at room temperature, a 21% overall yield (relative to the cyclohexane) of cyclohexanol and cyclohexanone was obtained with an overall turnover number (TON) of 213 (3) (71 per Co atom) or 10 (2) moles of products per mole of catalyst precursor (entries 3 and 19, respectively, Table 2).
The formation of CyOOH (under the conditions of Table 2) is proved by using the method proposed by Shul'pin.6d,10 The addition of PPh3 prior to the GC analysis of the products results in a marked increase of the amount of cyclohexanol (compare entries 1 and 11, Table 2) due to reduction of CyOOH by PPh3, with formation of phosphane oxide, and a corresponding decrease of cyclohexanone, as observed in other catalytic systems.11
The selectivity towards the cyclohexanol + cyclohexanone mixture is rather high, even for the high yields (e.g. 96% selectivity for 21% yield, entry 3, Table 2). This is much better than what obtained in the current industrial process (ca. 4% yield for ca. 85% selectivity),6a,h,12 in spite of the mild reaction conditions used in our case. Our yields are similar to reported for dinuclear CuII complexes11a (22–29%) or scorpionate CuII (ref. 11c) or CoIII,9c,11f complexes (up to 23%). As can be seen in Table 2, Cu(NO3)2, Co(NO3), [Cu(im)4](NO3)2 and [Co(im)6](NO3)2 provide only 4, 7, 8 and 9% total yield (entries 23–26), respectively. The high catalytic activity of 3 is expected to be due to its anionic part [C20H12CoIIIN8O8]− since the cationic part [Co(H2O)6]2+ can also be derived from Co(NO3)2 in solution, which provides low yield (entry 24, Table 2).
The catalytic performance of 2 and 3 under the same conditions, but replacing the oxidant by dioxygen, was assessed. It was found a negligible conversion of cyclohexane into the oxygenated products, thus suggesting the need of a peroxide oxidant, such as hydrogen peroxide, for this mild oxidative process.
The previously recognized common promoting effect of an acidic medium11d,f,13 on the peroxidative oxidation of alkanes is not observed for the present systems (Table 2). On the contrary, the presence of nitric acid has a drastic inhibiting effect on the catalytic activity of all the compounds tested (Fig. 2). Due to formation, in acidic medium, of stable a RAHB system in ligand molecules, complexes are not stable, i.e., protonation of ligands breaks down the active species (complex molecules) and therefore catalytic activity decreases. A similar behaviour was found for CuII complexes bearing azathia macrocycles14 and also for C-scorpionate AuIII complexes.15
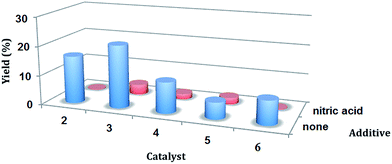 | ||
| Fig. 2 Catalytic activity of 2–6 for the oxidation of cyclohexane. Effect of the presence of nitric acid on the overall yield (cyclohexanol + cyclohexanone) using 2–6 as catalysts. | ||
The effect of the peroxide to catalyst molar ratio was also investigated (entries 1, 12–15, Table 2) and is depicted in Fig. 3. The increase of the peroxide amount up to n(H2O2)/n(catalyst) molar ratio of 2 × 103 leads to the maximum product yield and TON (Table 2, entry 1, Fig. 4). Further increase of the oxidant amount (up to 1 × 104) results in a strong yield drop (entry 15) eventually due to overoxidation reactions. In fact, for this oxidant to catalyst molar ratio, 1,4-cyclohexanedione was detected by CG-MS as the main overoxidation product (3.2% relative to cyclohexane). 1,3-Cyclohexanediol, 1,4-cyclohexanediol, 1,4-hydroxycyclohexanone, and 1,2-epoxycyclohexane were also detected, but in much lower amounts (1.4, 1.1, 0.3 and 0.2%, respectively, relative to cyclohexane).
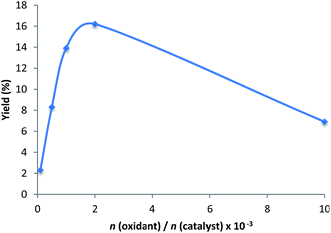 | ||
| Fig. 3 Effect of the molar H2O2 to catalyst ratio on the overall yield (cyclohexanol + cyclohexanone) using 2 as catalyst precursor. | ||
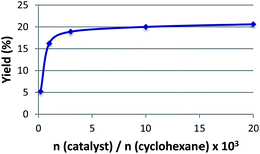 | ||
| Fig. 4 Effect of the molar catalyst to cyclohexane ratio on the overall yield (cyclohexanol + cyclohexanone) using 2 as catalyst. | ||
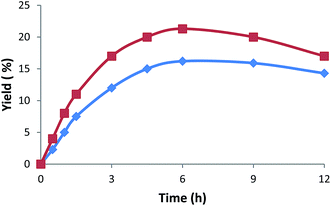
The activities of our catalytic systems are also dependent on the reaction time. Yields tend to increase on the extension of reaction time from up to 6 h (Fig. 5) whereafter a further increase of reaction time results in a yield drop due to subsequent reactions. In fact, overoxidation products such as 1,3-cyclohexanediol, 1,4-cyclohexanediol, 1,4-hydroxycyclohexanone, 1,4-cyclohexanedione or 1,2-epoxycyclohexane (e.g., 1.1, 0.8, 0.5, 0.9 or 0.3%, respectively, relative to cyclohexane, for 3) were detected by CG-MS for 9 h reaction time.
A noteworthy feature of the studied oxidations is the use of low catalyst loadings (typically 0.1 mol% vs. substrate) with high yields of oxygenated products (Table 2) considering the inertness of the substrate.
As observed for several metal catalytic systems,11,13,14 addition of a radical trap (CBrCl3 or Ph2NH) to the reaction mixture (Table 2, entries 20 and 21, respectively) results in a considerable suppression of the catalytic activity. This behaviour, along with the formation of cyclohexyl hydroperoxide (CyOOH, typical intermediate product in radical-type reactions) suggests a free-radical mechanism for cyclohexane oxidation, which will be further investigated.
Conclusions
In this study we have successfully used AHBA chelating ligands bearing barbituric acid fragment, as well as –OH and –SO3H groups in ortho-position of the aromatic part, and in several cases, imidazole (as an auxiliary ligand or a deprotonating agent for the weakening/destroying RAHB system in AHBA), to synthesis novel mononuclear CoII, CoII/III and CuII complexes. AHBA ligands play a crucial structural role in the organization of the H-bonded assemblies, influencing the overall 3D supramolecular arrangement (also depending on the metal ions) and imparting water solubility to the complexes.The CuII and CoII/III derivatives 2 and 3, respectively, display a high catalytic activity for the peroxidative (with H2O2) oxidation of cyclohexane in aqueous acetonitrile medium, under mild conditions.
Experimental
Materials and instrumentation
The 1H and 13C NMR spectra were recorded at room temperature on a Bruker Avance II + 300 (UltraShield™ Magnet) spectrometer operating at 300.130 and 75.468 MHz for proton and carbon-13, respectively. The chemical shifts are reported in ppm using tetramethylsilane as the internal reference. The infrared spectra (4000–400 cm−1) were recorded on a BIO-RAD FTS 3000MX instrument in KBr pellets. Carbon, hydrogen, and nitrogen elemental analyses were carried out by the Microanalytical Service of the Instituto Superior Técnico. Electrospray mass spectra (ESI-MS) were run with an ion-trap instrument (Varian 500 MS LC Ion Trap Mass Spectrometer) equipped with an electrospray ion source. For electrospray ionization, the drying gas and flow rate were optimized according to the particular sample with 35 p.s.i. nebulizer pressure. Scanning was performed from m/z 100 to 1200 in methanol solution. The compounds were observed in the negative or positive mode (capillary voltage = 80–105 V). The UV-vis absorption spectra in the 200–700 nm region were recorded with a scan rate of 240 nm min−1 by using a Lambda 35 UV-vis spectrophotometer (Perkin-Elmer) in 1.00 cm quartz cells at room temperature, with a concentration of H4L1 and 1–6 of 2.00 × 10−5 M in water. Chromatographic analyses were undertaken by using a Fisons Instruments GC 8000 series gas chromatograph with a DB-624 (J&W) capillary column (FID detector) and the Jasco-Borwin v.1.50 software. The internal standard method was used to quantify the organic products. The electrochemical experiments were performed on an EG&G PAR 273A potentiostat/galvanostat connected to a personal computer through a GPIB interface. Cyclic voltammetry (CV) studies were undertaken in 0.2 M [nBu4N][BF4]/MeCN, at a platinum disc working electrode (d = 0.5 mm) and at room temperature. Controlled-potential electrolyses (CPE) were carried out in electrolyte solutions with the above-mentioned composition, in a three-electrode H-type cell. The compartments were separated by a sintered glass frit and equipped with platinum gauze working and counter electrodes. For both CV and CPE experiments, a Luggin capillary connected to a silver wire pseudo-reference electrode was used to control the working electrode potential. A Pt wire was employed as the counter-electrode for the CV cell. The CPE experiments were monitored regularly by CV thus assuring no significant potential drift occurred along the electrolysis. The solutions were saturated with N2 by bubbling this gas before each run, the redox potentials of the complexes were measured by CV in the presence of ferrocene as the internal standard, and their values are quoted relative to the SCE by using the [Fe(η5-C5H5)2]0/+ redox couple (Eox1/2 = 0.42 V vs. SCE).9Syntheses of H4L1 and 1
The arylhydrazones of barbituric acid, 5-(2-(2-hydroxyphenyl)hydrazono)pyrimidine-2,4,6(1H,3H,5H)-trione (H4L1) and sodium salt of 2-(2-(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)hydrazinyl) benzenesulfonic acid (H4L2), [Na(H3L2)(μ-H2O)(H2O)2]2 (1), were synthesized according to the Japp–Klingemann reaction2,7 between the aromatic diazonium salt of substituted aniline and barbituric acid.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643 ν(C
O), 1643 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H), 1597 ν(C
O⋯H), 1597 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (300.130 MHz) in DMSO-d6, internal TMS, δ (ppm): 6.94–7.60 (4H, Ar–H), 10.62 (s, 1H, O–H), 11.23 (s, 1H, N–H), 11.44 (s, 1H, N–H), 14.44 (s, 1H, N–H). 13C{1H} NMR (75.468 MHz, DMSO-d6). δ: 115.10, 115.94, 117.79 and 120.09 (Ar–H), 126.82 (Ar–NHN = ), 129.09 (C
N) cm−1. 1H NMR (300.130 MHz) in DMSO-d6, internal TMS, δ (ppm): 6.94–7.60 (4H, Ar–H), 10.62 (s, 1H, O–H), 11.23 (s, 1H, N–H), 11.44 (s, 1H, N–H), 14.44 (s, 1H, N–H). 13C{1H} NMR (75.468 MHz, DMSO-d6). δ: 115.10, 115.94, 117.79 and 120.09 (Ar–H), 126.82 (Ar–NHN = ), 129.09 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 146.63 (Ar–OH), 149.86 and 160.01 (C
N), 146.63 (Ar–OH), 149.86 and 160.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 162.50 (C
O), 162.50 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H).
O⋯H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1604 ν(C
O), 1604 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H), 1520 ν(C
O⋯H), 1520 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 1H NMR (300.130 MHz) in DMSO-d6, internal TMS, δ (ppm): 7.21–7.80 (4H, Ar–H), 11.24 (s, 1H, N–H), 11.35 (s, 1H, N–H), 14.80 (s, 1H, N–H). 13C{1H} NMR (75.468 MHz, DMSO-d6). δ: 116.21, 118.25, 125.01 and 127.59 (Ar–H), 130.53 (Ar–NHNC
N) cm−1. 1H NMR (300.130 MHz) in DMSO-d6, internal TMS, δ (ppm): 7.21–7.80 (4H, Ar–H), 11.24 (s, 1H, N–H), 11.35 (s, 1H, N–H), 14.80 (s, 1H, N–H). 13C{1H} NMR (75.468 MHz, DMSO-d6). δ: 116.21, 118.25, 125.01 and 127.59 (Ar–H), 130.53 (Ar–NHNC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 135.86 (C
), 135.86 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 138.17 (Ar–SO3Na), 150.11 and 160.32 (C
N), 138.17 (Ar–SO3Na), 150.11 and 160.32 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.74 (C
O), 160.74 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H). The crystals of 1 suitable for X-ray structural analysis were obtained by slow evaporation of a methanol solution of the light orange powder.
O⋯H). The crystals of 1 suitable for X-ray structural analysis were obtained by slow evaporation of a methanol solution of the light orange powder.Syntheses of 2–6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v), then 136 mg (2 mmol) of imidazole (im) were added, under stirring. The addition of 232 mg (1 mmol) of Cu(NO3)2·2.5H2O together with 10 mL of distilled water resulted in a clear solution. The resulting solution was allowed to stand at room temperature and brown crystals of 2 were obtained after 1 day.
4, v/v), then 136 mg (2 mmol) of imidazole (im) were added, under stirring. The addition of 232 mg (1 mmol) of Cu(NO3)2·2.5H2O together with 10 mL of distilled water resulted in a clear solution. The resulting solution was allowed to stand at room temperature and brown crystals of 2 were obtained after 1 day.2: Yield, 229 mg, 51% (based on H4L1). It is soluble in water, DMSO, methanol, DMF, acetone and acetonitrile. Anal. calcd for C13H18CuN6O8 (Mr = 449.86): C, 34.71; H, 4.03; N, 18.68%. Found: C, 34.45; H, 3.95; N, 18.44%. IR (KBr): 3424 (s br) ν(H2O), 3060, 2870 and 2740 ν(NH), 1714 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1586 ν(C
O), 1586 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. ESI-MS: m/z: 310.7 [C10H6CuN4O4 + H]+.
N) cm−1. ESI-MS: m/z: 310.7 [C10H6CuN4O4 + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v), then 200 μL of triethylamine (Et3N) and 291 mg (1 mmol) of Co(NO3)2·6H2O were added at room temperature. The mixture was stirred for 30 min and left standing for slow solvent evaporation. Red crystals of 3 suitable for X-rays started to form in the reaction mixture after 1 day at room temperature; after 2 d they were filtered off and dried in air.
4, v/v), then 200 μL of triethylamine (Et3N) and 291 mg (1 mmol) of Co(NO3)2·6H2O were added at room temperature. The mixture was stirred for 30 min and left standing for slow solvent evaporation. Red crystals of 3 suitable for X-rays started to form in the reaction mixture after 1 day at room temperature; after 2 d they were filtered off and dried in air.3: Yield, 282 mg, 60% (based on Co). It is soluble in water, DMSO, methanol, DMF, acetonitrile and acetone. Anal. calcd for C40H52Co3N16O30 (Mr = 1413.73): C, 33.98; H, 3.71; N, 15.85%. Found: C, 33.66; H, 3.80; N, 15.67%. IR (KBr): 3451 (s br) ν(H2O), 3073, 2875 and 2733 ν(NH), 1719 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1594 ν(C
O), 1594 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. ESI-MS: m/z: 167.0 [Co(H2O)6]2+ and 551.2 [C20H12CoN8O8]−.
N) cm−1. ESI-MS: m/z: 167.0 [Co(H2O)6]2+ and 551.2 [C20H12CoN8O8]−.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v), then 291 mg (1.0 mmol) of Co(NO3)2·6H2O (232 mg of Cu(NO3)2·2.5H2O in the case of 5) and 204 mg (3.0 mmol) im (136 mg im in the case of 5) were added. The mixture was stirred for 5 min at 80 °C, then left for slow evaporation. Black (green in the case of 5) crystals of the product started to form after ca. 2 d at room temperature; they were then filtered off and dried in air.
4, v/v), then 291 mg (1.0 mmol) of Co(NO3)2·6H2O (232 mg of Cu(NO3)2·2.5H2O in the case of 5) and 204 mg (3.0 mmol) im (136 mg im in the case of 5) were added. The mixture was stirred for 5 min at 80 °C, then left for slow evaporation. Black (green in the case of 5) crystals of the product started to form after ca. 2 d at room temperature; they were then filtered off and dried in air.4: Yield, 298 mg, 52% (based on Co). It is soluble in water, DMSO, methanol and DMF. Anal. calcd for C19H18CoN10O6S (Mr = 573.41): C, 39.80; H, 3.16; N, 24.43%. Found: C, 39.74; H, 3.05; N, 24.32%. IR (KBr): 3380, 3220 and 2801 ν(NH), 1738 and 1664 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 ν(C
O), 1589 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. ESI-MS: m/z: 574.0 [Mr + H]+.
N) cm−1. ESI-MS: m/z: 574.0 [Mr + H]+.
5: Yield, 290 mg, 55% (based on Cu). It is soluble in water, DMSO, methanol and DMF. Anal. calcd for C16H16CuN8O7S (Mr = 527.96): C, 36.40; H, 3.05; N, 21.22%. Found: C, 36.14; H, 3.00; N, 21.14%. IR (KBr): 3454 ν(OH), 3145, 3075, 2980, 2892 and 2797 ν(NH), 1720, 1633 and 1606 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1521 ν(C
O), 1521 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. ESI-MS: m/z: 510.0 [Mr − H2O + H]+.
N) cm−1. ESI-MS: m/z: 510.0 [Mr − H2O + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v), then 200 μL of triethylamine (Et3N) and 291 mg (1 mmol) of Co(NO3)2·6H2O were added and the system heated to 80 °C for 10 min and left for slow evaporation. Red crystals of 7 started to form after ca. 2 d at room temperature; they were then filtered off and dried in air.
4, v/v), then 200 μL of triethylamine (Et3N) and 291 mg (1 mmol) of Co(NO3)2·6H2O were added and the system heated to 80 °C for 10 min and left for slow evaporation. Red crystals of 7 started to form after ca. 2 d at room temperature; they were then filtered off and dried in air.7: Yield, 229 mg, 49% (based on Co). It is soluble in water, DMSO, methanol, DMF, acetonitrile and acetone. Anal. calcd for C20H42CoN8O26S2 (Mr = 933.65): C, 25.73; H, 4.53; N, 12.00%. Found: C, 25.75; H, 4.42; N, 11.95%. IR (KBr): 3455 ν(OH), 3064, 2872 and 2677 ν(NH), 1738, 1675 and 1580 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1520 ν(C
O), 1520 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. ESI-MS: m/z: 312.1 [H3L3 + H]+ and 682.1 [Co(H3L3)2 + H]+.
N) cm−1. ESI-MS: m/z: 312.1 [H3L3 + H]+ and 682.1 [Co(H3L3)2 + H]+.
X-ray structure determinations
X-ray quality single crystals of the compounds were mounted in a nylon loop and measured at 150 K (1 and 3) or at room temperature (2 and 4–6). Intensity data were collected using a Bruker AXS-KAPPA APEX II diffractometer or a Bruker APEX-II PHOTON 100 diffractometer with graphite monochromated Mo-Kα (λ 0.71069) radiation. Data were collected using phi and omega scans of 0.5° per frame and a full sphere of data was obtained. Cell parameters were retrieved using Bruker SMART16a software and refined using Bruker SAINT16a on all the observed reflections. Absorption corrections were applied using SADABS.16a Structures were solved by direct methods by using the SHELXS package16b and refined with SHELXL-2014.16b Calculations were performed using the WinGX System-Version 1.80.03.16c The hydrogen atoms of water, hydrazine or ammonium groups were found in the difference Fourier map and the isotropic thermal parameters were set at 1.5 times the average thermal parameters of the belonging oxygen or nitrogen atoms, frequently with their distances restrained by using the DFIX and DANG commands. Coordinates of hydrogen atoms bonded to carbon atoms were included in the refinement using the riding-model approximation with the Uiso(H) defined as 1.2Ueq of the parent aromatic or methylene atoms. There were disordered molecules present in the structure of 4. Since no obvious major site occupations were found for those molecules, it was not possible to model them. PLATON/SQUEEZE16d was used to correct the data. A total of 136 electrons were estimated but the assignment is dubious. Thus, they were simply removed from the model. Least square refinements with anisotropic thermal motion parameters for all the non-hydrogen atoms and isotropic ones for the remaining atoms were employed. ESI.†Oxidation of cyclohexane
Typical reaction mixtures were prepared as follows: to 0.5–20.0 μmol of the compound H4L1 and 1–6 contained in the reaction flask were added 3 mL of MeCN, 5.00 mmol of C6H12 and 10 mmol of H2O2 solution (30% in H2O). In the experiments with radical traps, CBrCl3 (5.00 mmol) or NHPh2 (5.00 mmol) was added to the reaction mixture. The reaction mixture was stirred for 6 h at room temperature (ca. 298 K) and air atmospheric pressure, then 90 μL of cycloheptanone (as internal standard) and 5.0 mL of diethyl ether (to extract the substrate and the products from the reaction mixture) were added. The resulting mixture was stirred for 15 min and then a sample taken from the organic phase was analysed by GC. Subsequently, PPh3 was added to the final organic phase (to reduce the cyclohexyl hydroperoxide), the mixture was analysed again to estimate the amount of cyclohexyl hydroperoxide, according to Shul'pin's method.10 Blank experiments were performed and confirmed that no cyclohexane oxidation products were obtained in the absence of the metal catalyst precursor.Acknowledgements
This work has been partially supported by the Foundation for Science and Technology (FCT), Portugal (UID/QUI/00100/2013 program). The authors acknowledge the Portuguese NMR Network (IST-UTL Centre) for access to the NMR facility, and the IST Node of the Portuguese Network of mass-spectrometry (Dr Conceição Oliveira) for the ESI-MS measurements. The authors also acknowledge the financial support by the University of Camerino (Fondo di Ateneo per la Ricerca 2011–2012) and Nuova Simonelli Company.References
- (a) A. Slaczka and J. Lubczak, J. Appl. Polym. Sci., 2007, 106, 4067–4074 CrossRef CAS PubMed; (b) D. Thetford, A. P. Chorlton and J. Hardman, Dyes Pigm., 2003, 59, 185–191 CrossRef CAS; (c) M. R. Zamanloo, A. Shamkhali, M. Alizadeh, Y. Mansoori and G. Imanzadeh, Dyes Pigm., 2012, 95, 587–599 CrossRef CAS PubMed; (d) M. Tishler, K. Pfister, R. D. Babson, K. Ladenburg and A. J. Fleming, J. Am. Chem. Soc., 1947, 69, 1487–1492 CrossRef CAS.
- K. T. Mahmudov, M. N. Kopylovich, A. M. Maharramov, M. M. Kurbanova, A. V. Gurbanov and A. J. L. Pombeiro, Coord. Chem. Rev., 2014, 265, 1–37 CrossRef CAS PubMed.
- (a) E. Colacio, J. Ruiz, J. M. Moreno, R. Kivekas, M. R. Sundberg, J. M. Dominguez-Vera and J. P. Laurent, J. Chem. Soc., Dalton Trans., 1993, 157–163 RSC; (b) E. Colacio, M. Quiros, J. M. Salas, A. Garca and J. D. Martin, J. Crystallogr. Spectrosc. Res., 1993, 23, 407–410 CrossRef; (c) E. Colacio, J.-M. Dominguez-Vera, R. Kivekas and J. Ruiz, Inorg. Chim. Acta, 1994, 218, 109–119 CrossRef CAS; (d) E. Colacio, J.-M. Dominguez-Vera, J.-P. Costes, R. Kivekas, J.-P. Laurent, J. Ruiz and M. Sundberg, Inorg. Chem., 1992, 31, 774–778 CrossRef CAS; (e) E. Colacio, J.-M. Dominguez-Vera, R. Kivekas, J. M. Moreno and J. Ruiz, Inorg. Chim. Acta, 1994, 219, 127–133 CrossRef CAS; (f) J. M. Moreno, J. Ruiz, J.-M. Dominguez-Vera, E. Colacio, D. Galisteo and R. Kivekas, Polyhedron, 1994, 13, 203–207 CrossRef CAS; (g) V. Sadasivan and M. Alaudeen, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2007, 46, 1959–1964 Search PubMed; (h) K. T. Mahmudov, M. F. C. Guedes da Silva, M. Glucini, M. Renzi, K. C. P. Gabriel, M. N. Kopylovich, M. Sutradhar, F. Marchetti, C. Pettinari, S. Zamponi and A. J. L. Pombeiro, Inorg. Chem. Commun., 2012, 22, 187–189 CrossRef CAS PubMed.
- (a) M. N. Kopylovich, A. C. C. Nunes, K. T. Mahmudov, M. Haukka, T. C. O. Mac Leod, L. M. D. R. S. Martins, M. L. Kuznetsov and A. J. L. Pombeiro, Dalton Trans., 2011, 40, 2822–2836 RSC; (b) M. N. Kopylovich, K. T. Mahmudov, M. F. C. G. Silva, L. M. D. R. S. Martins, M. L. Kuznetsov, T. F. S. Silva, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, J. Phys. Org. Chem., 2011, 24, 764–773 CAS; (c) M. N. Kopylovich, M. F. C. Guedes da Silva, L. M. D. R. S. Martins, M. L. Kuznetsov, K. T. Mahmudov and A. J. L. Pombeiro, Polyhedron, 2013, 50, 374–382 CrossRef CAS PubMed.
- A. Mizar, M. F. C. Guedes da Silva, M. N. Kopylovich, S. Mukherjee, K. T. Mahmudov and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2012, 2305–2313 CrossRef CAS PubMed.
- (a) Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 6th edn, 2002 Search PubMed; (b) G. B. Shul'pin, Oxidations of C–H Compounds Catalyzed by Metal Complexes, in Transition Metals for Organic Synthesis, ed. M. Beller and C. Bolm, Wiley–VCH, Weinheim, New York, 2nd edn, 2004, vol. 2, pp. 215−242 Search PubMed; (c) G. B. Shul'pin, Org. Biomol. Chem., 2010, 8, 4217–4228 RSC; (d) G. B. Shul'pin, Dalton Trans., 2013, 42, 12794–12818 RSC; (e) A. E. Shilov and G. B. Shul'pin, Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2000 Search PubMed; (f) M. N. Kopylovich, T. C. O. Mac Leod, M. Haukka, G. I. Amanullayeva, K. T. Mahmudov and A. J. L. Pombeiro, J. Inorg. Biochem., 2012, 115, 72–77 CrossRef CAS PubMed; (g) M. N. Kopylovich, M. J. Gajewska, K. T. Mahmudov, M. F. C. Guedes da Silva, M. V. Kirillova, P. J. Figiel, J. Sanchiz and A. J. L. Pombeiro, New J. Chem., 2012, 36, 1646–1654 RSC; (h) U. Schuchardt, D. Cardoso, R. Sercheli, R. Pereira, R. S. da Cruz, M. C. Guerreiro, D. Mandelli, E. V. Spinacé and E. L. Pires, Appl. Catal., A, 2001, 211, 1–17 CrossRef CAS; (i) M. Sutradhar, N. V. Shvydkiy, M. F. C. Guedes da Silva, M. V. Kirillova, Y. N. Kozlov, A. J. L. Pombeiro and G. B. Shul'pin, Dalton Trans., 2013, 42, 11791–11803 RSC; (j) M. Sutradhar, L. M. D. R. S. Martins, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Coord. Chem. Rev., 2015, 301–302, 200–239 CrossRef CAS PubMed; (k) K. M. Parida, M. Sahoo and S. Singha, J. Mol. Catal. A: Chem., 2010, 329, 7–12 CrossRef CAS PubMed.
- (a) A. M. Maharramov, R. A. Aliyeva, I. A. Aliyev, F. G. Pashaev, A. G. Gasanov, S. I. Azimova, R. K. Askerov, A. V. Kurbanov and K. T. Mahmudov, Dyes Pigm., 2010, 85, 1–6 CrossRef CAS PubMed; (b) K. T. Mahmudov, A. M. Maharramov, R. A. Aliyeva, I. A. Aliyev, R. K. Askerov, R. Batmaz, M. N. Kopylovich and A. J. L. Pombeiro, J. Photochem. Photobiol., A, 2011, 219, 159–165 CrossRef CAS PubMed; (c) S. R. Gadjieva, T. M. Mursalov, K. T. Mahmudov and F. M. Chyragov, Russ. J. Coord. Chem., 2006, 32, 304–308 CrossRef; (d) K. T. Mahmudov, M. N. Kopylovich and A. J. L. Pombeiro, Coord. Chem. Rev., 2013, 257, 1244–1281 CrossRef CAS PubMed; (e) K. T. Mahmudov, R. A. Aliyeva, S. R. Gadjieva and F. M. Chyragov, J. Anal. Chem., 2008, 63, 435–438 CrossRef.
- (a) A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC; (b) K. Robinson, G. V. Gibbs and P. H. Ribbe, Science, 1971, 172, 567–570 CAS.
- (a) A. J. L. Pombeiro, M. F. C. Guedes da Silva and M. A. N. D. A. Lemos, Coord. Chem. Rev., 2001, 219–221, 53–80 CrossRef CAS; (b) M. E. N. P. R. A. Silva, A. J. L. Pombeiro, J. J. R. Fraústo da Silva, R. Herrmann, N. Deus, T. J. Castilho and M. F. C. Guedes da Silva, J. Organomet. Chem., 1991, 421, 75–90 CrossRef CAS; (c) A. I. F. Venâncio, M. L. Kuznetsov, M. F. C. Guedes da Silva, L. M. D. R. S. Martins, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, Inorg. Chem., 2002, 41, 6456–6467 CrossRef PubMed; (d) A. I. F. Venâncio, M. F. C. Guedes da Silva, L. M. D. R. S. Martins and A. J. L. Pombeiro, Organometallics, 2005, 24, 4654–4665 CrossRef.
- (a) G. B. Shul'pin, C. R. Chim., 2003, 6, 163–178 CrossRef; (b) G. B. Shul'pin, Y. N. Kozlov, L. S. Shul'pina, A. R. Kudinov and D. Mandelli, Inorg. Chem., 2009, 48, 10480–10482 CrossRef PubMed; (c) G. B. Shul'pin, Y. N. Kozlov, L. S. Shul'pina and P. V. Petrovskiy, Appl. Organomet. Chem., 2010, 24, 464–472 Search PubMed; (d) G. B. Shul'pin, J. Mol. Catal. A: Chem., 2002, 189, 39–66 CrossRef.
- (a) A. M. Kirillov, M. V. Kirillova and A. J. L. Pombeiro, Coord. Chem. Rev., 2012, 256, 2741–2759 CrossRef CAS PubMed; (b) L. M. D. R. S. Martins and A. J. L. Pombeiro, in Advances in Organometallic Chemistry and Catalysis, ed. A. J. L. Pombeiro, Wiley–VCH, Weinheim, 2013, ch. 22, pp. 285–294 Search PubMed; (c) L. M. D. R. S. Martins and A. J. L. Pombeiro, Coord. Chem. Rev., 2014, 265, 74–88 CrossRef CAS PubMed; (d) M. N. M. Milunovic, L. M. D. R. S. Martins, E. C. B. A. Alegria, A. J. L. Pombeiro, R. Krachler, G. Trettenhahn, C. Turta, S. Shova and V. B. Arion, Dalton Trans., 2013, 42, 14388–14401 RSC; (e) M. Sutradhar, M. V. Kirillova, M. F. C. Guedes da Silva, L. M. D. R. S. Martins and A. J. L. Pombeiro, Inorg. Chem., 2012, 51, 11229–11231 CrossRef CAS PubMed; (f) T. F. S. Silva, L. M. D. R. S. Martins, M. F. C. Guedes da Silva, M. L. Kuznetsov, A. R. Fernandes, A. Silva, S. Santos, C.-J. Pan, J.-F. Lee, B.-J. Hwang and A. J. L. Pombeiro, Chem.–Asian J., 2014, 9, 1132–1143 CrossRef CAS PubMed; (g) T. F. S. Silva, M. F. C. G. Silva, G. S. Mishra, L. M. D. R. S. Martins and A. J. L. Pombeiro, J. Organomet. Chem., 2011, 696, 1310–1318 CrossRef CAS PubMed.
- K. Weissermel and H. J. Arpe, in Industrial Organic Chemistry, VCH Press, Weinheim, 2nd edn, 1993 Search PubMed.
- M. V. Kirillova, Y. N. Kozlov, L. S. Shul'pina, Q. Y. Lyakin, A. M. Kirillov, E. P. Talsi, A. J. L. Pombeiro and G. B. Shul'pin, J. Catal., 2009, 268, 26–38 CrossRef CAS PubMed.
- (a) R. R. Fernandes, J. Lasri, A. M. Kirillov, M. F. C. Guedes da Silva, J. A. L. Silva, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2011, 3781–3790 CAS; (b) R. R. Fernandes, J. Lasri, M. F. C. Guedes da Silva, J. A. L. da Silva, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, J. Mol. Catal. A: Chem., 2011, 351, 100–111 CrossRef CAS PubMed.
- M. Peixoto de Almeida, L. M. D. R. S. Martins, S. A. C. Carabineiro, T. Lauterbach, F. Rominger, A. S. K. Hashmi, A. J. L. Pombeiro and J. L. Figueiredo, Catal. Sci. Technol., 2013, 3, 3056–3069 CAS.
- (a) APEX2 & SAINT, Bruker AXS Inc., Madison, WI, 2004 Search PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed; (c) L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837–838 CrossRef CAS; (d) A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, C34 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal data, experimental parameters and selected details of the refinement calculations, cyclic voltammogram (for H4L1 and 1–3) and electron absorption spectra of compounds 1–6. CCDC 1055317, 1055319–1055322 and 1055325. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14078a |
| This journal is © The Royal Society of Chemistry 2015 |