An experimental and theoretical study on the regioselective synthesis of a new class of spiropyrrolothiazoles with quinoxaline motifs via a 1,3-dipolar cycloaddition reaction. An evaluation of DFT methods†
Mahshid Hamzehloueian*a,
Yaghoub Sarrafib and
Zahra Aghaeib
aDepartment of Chemistry, Jouybar Branch, Islamic Azad University, Jouybar, Iran. E-mail: m.hamzehlooian@gmail.com
bDepartment of Organic Chemistry, Faculty of Chemistry, University of Mazandaran, 47416 Babolsar, Iran
First published on 1st September 2015
Abstract
A series of novel spiropyrrolothiazoles with quinoxalines motifs have been synthesized by a four-component 1,3-dipolar cycloaddition reaction of various derivatives of trans-β-nitrostyrene and an azomethine ylide, generated in situ from 1,3-thiazolane-4-carboxylic acid, ninhydrin and 1,2-phenylenediamine. The stereochemistry of the products has been confirmed by single crystal X-ray structure and spectroscopic techniques. Theoretical calculations have been carried out using DFT methods at the B3LYP/6-31G(d,p), wB97xD/6-31G(d,p) and M06-2X/6-31G(d,p) levels. The regio- and stereoselectivity have been explained on the basis of transition state stabilities and global and local reactivity indices of the reactants. The assessment of geometries and energetics of transition states revealed the importance of π/π interactions between aromatic rings in the regioselectivity of the cycloaddition reaction. The B3LYP functional is unable to deal with this weak interaction in the proposed TSs and it leads to an incorrect conclusion about the reaction regioselectivity. In this report, we successfully employed wB97xD and M06-2X functionals to calculate the transition states relative energies.
Introduction
The 1,3-dipolar cycloaddition (1,3-DC) reaction of azomethine ylides with dipolarophiles is an efficient method for the construction of nitrogen-containing five membered heterocycles in a highly regio- and stereo-selective manner.1 Azomethine ylides can be produced by a number of methods, of which the decarboxylation route is a facile manner and was investigated extensively by Grigg and co-workers.2 Among the various nitrogen containing heterocycles, pyrrolidines, pyrrolizidines and pyrrolothiazoles have gained much consideration due to their unique biological activities, i.e. antimicobacterial,3 hepatoprotective,4 antibiotic,5 antidiabetic6 and anticonvulsant actions.7 They also form the central skeleton of many natural products and alkaloids.8 Spiropyrrolothiazoles have shown diverse biological activities such as antimicrobial,9 antidiabetic,10 anticancer11 and acetylcholinesterase-inhibitory properties.12 It is interesting to note from chemical literature that benzopyrazines (quinoxalines) and their derivatives also exhibit spectrum of biological activities like antimicrobial, antifungal, anticancer and anthelmintic properties.13 The other applications of this important motif includes dying, synthesis of pharmaceuticals and organic semi-conductors.14Based on the biological activities of these derivatives and in continuation of our previous theoretical and experimental research program on the spiro heterocycles,15 we became interested in the synthesis of a novel class of spiro-pyrrolothiazoles with quinoxalines motifs via the one-pot, four-component condensation of trans-β-nitrostyrene derivatives with an azomethine ylide, generated in situ from 1,3-thiazolane-4-carboxylic acid, ninhydrin and 1,2-phenylenediamine. In particular, we focused our attention to study of mechanism and regioselectivity of this 1,3-DC reaction from a theoretical point of view by means of the Density Functional Theory (DFT).
Results and discussion
In our initial endeavor, we have investigated a four-component reaction of trans-β-nitrostyrene 4a as dipolarophile with a non-stabilized azomethine ylide, generated in situ by the decarboxylative condensation of 1,3-thiazolane-4-carboxylic acid 3, ninhydrin 1 and 1,2-phenylenediamine 2 in refluxing ethanol to give a single cycloadduct 5a (Scheme 1). The extent of reaction was monitored by TLC and the pure cycloadduct was obtained by recrystallization from ethanol. This protocol was applied to a series of various derivatives of nitroalkene 4b–n with both electron-withdrawing and electron-releasing substituents under similar conditions (Scheme 1). We found no spectroscopic evidence for a competing formation of isomeric compounds 6.The structure and regiochemistry of the cycloadducts were determined by various spectroscopic techniques. So, the IR spectrum of 5c demonstrated absorption at 1338 and 1543 cm−1 indicating the presence of a nitro group. The 1H NMR spectrum of 5c exhibited a singlet at δ = 3.5 for the methoxy protons, a doublet at δ = 4.69 ppm (J = 11.2 Hz) for Ha, a doublet of doublet and not a doublet at δ = 6.82 ppm (J = 11.4, 7.4 Hz) for Hb (CHNO2) and a quartet at δ = 4.86 ppm (J = 7.2 Hz) for Hc. This suggests that the correct regiochemistry of the product is 5c as shown in Scheme 1. The 13C NMR of 5c showed a peak at δ = 75.28 ppm owing to the spiro carbon and a peak at δ = 86.06 ppm for the carbon attached to the nitro group. The formation of the product was also confirmed by mass spectral. The mass spectrum of 5c showed a molecular ion peak at 482 (M+). Identical consequence were obtained for other products.
The ORTEP view of single crystal X-ray analysis of 5c is indicated in Fig. 1.16 The stereochemistry of cycloadducts is compatible with an S-shaped ylide and following cycloaddition by an endo-transition state (pathway B, Scheme 2).
Computational details
DFT calculations were done using the B3LYP, wB97xD and M06-2X functionals and the 6-31G(d,p) basis set, in the Gaussian 09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 environment. The electronic structures of critical points were surveyed by the natural bond orbital (NBO) way.18 The nature of stationary geometries has been characterized by calculating the frequencies in order to verify that the transition states have only one imaginary frequency with the corresponding eigenvector involving the formation of the newly created C–C bonds. Also, zero-point energies and thermodynamic modifications were obtained using analytical force stable. Thermal modifications to enthalpy and entropy values were measured at 298.15 K.
17 environment. The electronic structures of critical points were surveyed by the natural bond orbital (NBO) way.18 The nature of stationary geometries has been characterized by calculating the frequencies in order to verify that the transition states have only one imaginary frequency with the corresponding eigenvector involving the formation of the newly created C–C bonds. Also, zero-point energies and thermodynamic modifications were obtained using analytical force stable. Thermal modifications to enthalpy and entropy values were measured at 298.15 K.
Prediction of regiochemistry
Due to the asymmetry of both the dipole and dipolarophile, the 1,3-dipolar cycloaddition reaction between nitrostyrene 4a and azomethine ylide 7 can yield four isomeric products. First, we investigated the reaction energy path using B3LYP/6-31G(d,p). Four possible transition structures and related products have been optimized and characterized. They are related to two regioisomeric and two stereoisomeric approaches (Scheme 3).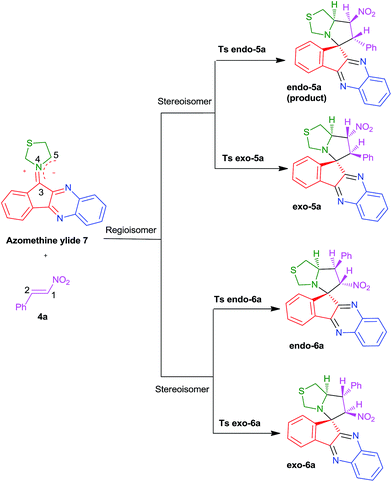 | ||
| Scheme 3 Regio- and stereoisomeric pathways for a 1,3-DC reaction between azomethine ylide 7 with trans-β-nitrostyrene 4a. | ||
The optimized geometries of transition states are represented in Fig. 2. The activation energies, enthalpies and Gibbs free energies as well as the reaction energies, enthalpies, and Gibbs free energies are reported in Table S1.†
In recent years, nucleus independent chemical shift (NICS)19 values have been extensively used to assess aromaticity.19,20 In order to evaluate the aromaticity of the optimized transition states, NICS was computed using gauge invariant atomic orbital (GIAO)21 approach at the B3LYP/6-31G(d,p). As shown in Fig. 2, all of the considered transition structures have large negative NICS values that indicate the aromatic characters of TSs due to the six electrons undergoing bond changing. In contrast, all cycloadducts have small negative NICS values (Table S2†). Therefore, it can be concluded that possible transition states associated with 1,3-DC reaction between azomethine ylide 7 and β-nitrostyrene 4a exhibit in-plane aromaticity.
As displayed in Fig. 2, in the gas phase, the energy of TS endo-6a is 1.65 kcal mol−1 lower than that of TS endo-5a, favoring the formation of the endo-6a regioisomer as a product. One can see that the B3LYP functional fails to estimate the observed regioselectivity of the reaction. We find that the benzene ring in nitrostyrene and aromatic part of azomethine ylide are stacked in TS endo-5a, therefore there could be a dispersion π/π weak interaction between them which can be the reason of making the TS endo-5a more energy favorable.
The role of dispersion is very important in controlling stereo-selectivities of many reactions.22 In our previous theoretical study,15b we reported that π/π interactions play a major role in the regiochemistry of a 1,3-DC reaction. It is well known that B3LYP is not able to estimate the stabilization gained by π/π interactions,23 so we were encouraged to study the regiochemistry with more appropriate functional.
There are two approaches to deal with the weak interactions. One rout involves using post Hartree–Fock methods such as Møller–Plesset perturbation (MP2, MP4), coupled cluster and Configuration Interaction (CI) methods. All of this methods require huge computational resources which is not readily available at this time for molecules as large as studied here. The other popular strategy is to augment conventional DFT functionals with ad hoc addition of non-bonded pairwise interaction namely DFT-D methods which contain a large number of free parameters in the functional form which are semiempirically fit using diverse data sets that include thermochemistry, noncovalent interactions and kinetics data.24 So, the deficiency of hybrid functionals in the treatment of dispersion, can be minimized. The long-range van der Waals interaction will be included through the semi-empirical Grimme correction which does not attempt to describe the actual source of the interaction (fluctuating dipoles) but rather its effect on the DFT mean-field effective potential. The wB97xD and M06-2x functionals are two of the most popular meta GGA functionals in computational chemistry. The functional wB97xD is a version of Grimme's D2 dispersion model which is the latest functional from Head-Gordon and coworkers.25 According to Zhao and Truhlar, the M05 and M06 series of functionals implicitly account for “medium-range” electron correlation because of the way they are parametrized, and this is sufficient to describe the dispersion interactions within many complexes.26
It is worth mentioning that, theoretical investigations to understand the accuracy of these functionals in the prediction of regioselectivity in cycloaddition reactions is rare.27 Four possible transition structures have been optimized and characterized using wB97xD/6-31G(d,p) and M06-2X/6-31G(d,p). As shown in Table 1 in both level of theories, TS endo-5a has the lowest energy compared to other transition state isomers as a result of the π/π interaction, in accordance with experiment. Both method estimate that the relative stabilization of possible stationary points are: TS endo-5a > TS endo-6a > TS exo 5a > TS exo-6a (Fig. 3).
| Structure | Erela | Erelb | CT | ΔR | δBav | Sy | ΔH |
|---|---|---|---|---|---|---|---|
| a Relative to the most stable TS (TS endo-5a).b Relative to reagents (4a+7). | |||||||
| TS endo-5a | 0.00 | −12.75 | 0.2580 | 0.168 | 0.32 | 0.78 | −52.18 |
| (0.00) | (−13.89) | (0.2729) | (0.289) | (0.31) | (0.75) | (−56.76) | |
| TS exo-5a | 4.86 | −7.89 | 0.1749 | 0.348 | 0.24 | 0.51 | −46.80 |
| (5.01) | (−8.89) | (0.1669) | (0.379) | (0.24) | (0.51) | (−50.55) | |
| TS endo-6a | 2.34 | −10.41 | 0.2293 | 0.395 | 0.16 | 0.27 | −49.36 |
| (3.77) | (−10.12) | (0.2195) | (0.403) | (0.15) | (0.18) | (−52.87) | |
| TS exo-6a | 7.20 | −5.55 | 0.1953 | 0.468 | 0.21 | 0.21 | −50.08 |
| (8.33) | (−5.56) | (0.1989) | (0.420) | (0.19) | (0.16) | −53.28 | |
The calculated activation energies with respect to the sum of the energies of the separated dipole 7 and dipolarophile 4a are negative (Table 2). It is well understood that as two separated reactants are approached, a van der Waals pre-complex which is energetically more stable than the separated reactants, is initially formed between them in an appropriate distance and TS is formed from this pre-complex instead of separated reactants.28
| Structure | μ (a.u) | η (a.u) | ω (eV) | s |
|---|---|---|---|---|
| Nitrostyrene 4a | −0.182 | 0.247 | 1.82 | 2.028 |
| (−0.176) | (0.158) | (2.66) | (3.152) | |
| Azomethine ylide 7 | −0.121 | 0.183 | 1.10 | 2.732 |
| (−0.119) | (0.111) | (1.73) | (4.474) |
The charge transfer (CT) was calculated for all the optimized TSs, in terms of the residual charge on the azomethine ylide 7, using Natural Population Analysis (NPA) at B3LYP, M06-2X and wB97xD levels (see Tables S1† and 1). The positive values are indicative of an electron flow from the HOMO of the azomethine ylide 7 to the LUMO of the nitrostyrene 4a. These values indicate that these TSs have some zwitterionic character. The CT for the most favourable TSs, TS endo-5a at M06-2X (wB97xD) and TS endo-6a at B3LYP, is higher than the others.
The B3LYP/6-31G(d,p) lengths of the C2–C3 and C1–C5 forming-bonds are 2.40 and 2.17 Å at the TS endo-5a and 2.72 and 2.08 Å at TS exo-5a, respectively, while the lengths of the C1–C3 and C2–C5 forming bonds are 2.73 and 2.13 Å at TS endo-6a and 2.70 and 2.09 Å at TS exo-6a, respectively (Fig. 2). The M06-2x/6-31G(d,p) geometries of the TSs involved in this 1,3-DC reaction are given in Fig. 3. The M06-2x/6-31G(d,p) lengths of the C2–C3 and C1–C5 forming-bonds are 2.18 and 2.35 Å at the TS endo-5a and 2.51 and 2.16 Å at TS exo-5a, respectively, while the lengths of the C1–C3 and C2–C5 forming bonds are 2.59 and 2.19 Å at TS endo-6a and 2.60 and 2.14 Å at TS exo-6a, respectively. A comparison of the B3LYP and M06-2X geometries for the 1,3-DC reaction, indicates that both functionals yield similar bond-formation processes except for the TS endo-5a. Different results are obtained where the M06-2X functional affords earlier bond-formation processes in the spiro center. Thus, the length of the C2–C3 is smaller than C1–C5 at M06-2X while the reverse was the case for B3LYP. The wB97xD results are similar with M06-2X. This can be related to proper position of aromatic rings to allow the π/π interaction.
The difference between lengths of forming bonds in each TSs, ΔR, provides an estimate for the synchronicity of the bond-formation process. The calculated ΔR of TSs in Table 1 showed that reaction is asynchronous and TS endo-5a is more synchronous than other TSs.
The synchronicity (Sy) index of the cycloaddition reactions is estimated by the equation29
 | (1) |
 | (2) |
 | (3) |
According to eqn (1), for a perfectly synchronous process, Sy = 1 and for fully asynchronous process synchronicity is zero. Based on eqn (2) and (3), early and late transition structures will be characterized by δBav <0.5 and δBav >0.5, respectively.29g The computed synchronicities (Sy) for four possible channels at M06-2X/6-31G(d,p) show that synchronicities order is TS endo-5a > TS exo-5a > TS endo-6a > TS exo-6a. The δBavs are in all cases indicative of an early transition state which is in agreement with the exothermic nature of the reactions (Table 1).
To predict the regioselectivity of cycloaddition reaction, a study using FMO analysis31 and the global and local indices32 for electrophilic/nucleophilic was also carried out at M06-2X/6-31G(d,p). Two types of azomethine ylide, anti (s-shape) and syn (w-shape) can be resulted from the reaction of ninhydrin 1, 1,2-phenylenediamine 2 and 1,3-thiazolane-4-carboxylic acid 3 (Scheme 2). The s-shape azomethine ylide energy is lower than the w-shape by 1.41 kcal mol−1 which is in line with the product structure. In this study, the HOMO–LUMO energy gaps suggest that the HOMOdipole–LUMOdipolarophile interaction controls the cycloaddition reaction within a normal electron demand reaction33 (Fig. 4). Inspection of the LUMO coefficients of the nitrostyrene 4a and the HOMO coefficients of azomethine ylide 7 on the reactive sites in Table 3, revealed that the most favored large–large interaction takes place between C2 of the nitrostyrene and C3 of the azomethine ylide, which is in good agreement with the experimental observation.
 | ||
| Fig. 4 HOMO and LUMO energies of dipolarophile 4a and azomethine ylide 7 calculated at the M06-2X/6-31G(d,p) level. | ||
| Reactant | Site | HOMO coefficients | LUMO coefficients | fk− | fk+ | s+ | s− | ωk |
|---|---|---|---|---|---|---|---|---|
| Nitrostyrene 4a | C1 | 0.2909 | 0.2208 | 0.112 | 0.002 | 0.0041 | 0.2272 | 0.01 |
| (0.3090) | (0.1994) | (0.100) | (0.042) | (0.1325) | (0.3180) | (0.11) | ||
| C2 | 0.1458 | 0.2904 | 0.004 | 0.079 | 0.1602 | 0.0081 | 0.14 | |
| (0.1475) | (0.2971) | (0.013) | (0.070) | (0.2235) | (0.0437) | (0.18) | ||
| Azomethine ylide 7 | C3 | 0.3434 | 0.0263 | 0.123 | 0.015 | 0.0409 | 0.3361 | 0.016 |
| (0.3415) | (0.0543) | (0.102) | (0.013) | (0.0600) | (0.4563) | (0.02) | ||
| C5 | 0.2883 | 0.2325 | 0.099 | 0.042 | 0.1148 | 0.2705 | 0.046 | |
| (0.3092) | (0.3011) | (0.086) | (0.075) | (0.3375) | (0.3848) | (0.13) |
Electronic chemical potential is defined as the mean value of HOMO and LUMO energies [μ= (EHOMO + ELUMO)/2] and is a relative measure of the molecular capacity to donate electron density, while the global electrophilicity index is the ratio ω = μ2/(2η), which measures the total ability to attract electrons. η is the chemical hardness, and is the difference between the HOMO and LUMO energies [η=(ELUMO − EHOMO).34
The calculated global properties of dipole 7 and dipolarophile 4a are shown in Table 2. The global reactivity indices also supported HOMOdipole–LUMOdipolarophile interaction. The electrophilicity of nitrostyrene at M06-2X/6-31G(d,p) in Table 2 is greater than 1.5 eV, thus according to the classification of electrophilicity, these compounds can be classified as strong electrophiles.35a However, the electrophilicity of nitrostyrene (1.82 eV) is greater than that of the azomethine ylide (1.10 eV). The electronic chemical potentials, μ, of the azomethine ylide 7 (−0.121 a.u.) is higher than those for the nitrostyrene 4a, (−0.182 a.u.) indicating that along this 1,3-DC, azomethine ylide acts as a nucleophile whereas nitrostyrene acts as an electrophile and consequently the electronic flux is from dipole 7 to dipolarophile 4a. As shown in Table 2, the B3LYP results are in agreement with M06-2X.
Studies of the regioselectivity of 1,3-DC reactions have demonstrated that the analysis of the local electrophilicity, ωk, at the electrophile, together with the analysis of the nucleophilic Fukui functions, fk−, at the nucleophile, permit prediction of the regioselectivity in these competitive cycloadditions.35,36 To reveal the most favorable reactive site, the DFT-based local chemical reactivity Fukui function for nucleophilic (fk+) and electrophilic (fk−) attacks, have been calculated through the NBO (Natural Bond Orbital) and Mulliken charges analysis using eqn (4) and (5), respectively.
| fk+ = ρkN+1 − ρkN = qkN − qkN+1 | (4) |
| fk− = ρkN − ρkN−1 = qkN−1 − qkN | (5) |
As shown in Table 3, nitrostyrene 4a has the largest electrophilic activation at the C2 carbon atom, ωk = 0.14 eV, whereas the azomethine ylide 7 has the largest nucleophilic activation at the C3 carbon atom, fk− = 0.123. Therefore, C2 of nitrostyrene will be the preferred position for a nucleophilic attack by C3 of the azomethine ylide 7, which is in good agreement with the observed regioselectivity. The B3LYP results are in accordance with M06-2X (see Table 3).
Local softness, especially in conjunction with the hard and soft acids and bases (HSAB) principle, used successfully in predicting the regioselectivity of several cycloaddition reactions.38 This principle states that the most favorable interaction results when the reactants have equal softness. Local softness and the HSAB principle thus provide a way to predict the favored product on the basis of the electronic properties of the isolated reactants. Predominance of one regioisomer over the other can be quantified by softness matching index Δ.39
| Δklij = (si− − sk+)2 + (sj− − sl+)2 | (6) |
| sk± = fk±S | (7) |
 | (8) |
The reaction pathway involving a lower value of Δklij will be the favored one. For 1,3-DC of azomethine ylide 7 and nitrostyrene 4a, Δklij for the generation of 5a is smaller compared to that for 6a (0.102 vs. 0.122). This suggests a preference for the generation of 5 along the cycloaddition reaction and hence agrees well with the regioselectivity experimentally observed. The B3LYP results are in agreement with M06-2X (0.117 vs. 0.130 for regioisomer 5a compared with 6a).
Conclusions
In summary, we have developed an efficient and simple method to regioselective synthesis of novel spiropyrrolothiazoles with quinoxalines motifs via a one pot four-component 1,3-DC of an azomethine ylide with trans-β-nitrostyrene derivatives. The pure products were isolated by recrystallization without requiring a further purification step. Mechanism and the regiochemistry of the reaction have been studied in terms of global and local reactivity indices, FMO analysis and characterization of relevant transition states at the B3LYP, wB97xD and M06-2X level of theories. The calculated global and local reactivity indices, FMO analysis and HSAB results at wB97xD and M06-2X are in line with the B3LYP's one. Although, popular B3LYP functional failed to predict the observed regioselectivity from the calculated energy paths of possible channels, wB97xD and M06-2X functionals were applied successfully. So, one can conclude that application of DFT dispersion corrected methods is essential in examining the regioselectivities of reactions by energies of transition states.Experimental
General procedure for preparation of spiropyrrolothiazole 5(a–n)
A mixture of ninhydrin 1 (0.178 g, 1 mmol), 1,2-phenylenediamine 2 (0.108 g, 1 mmol), thiazolidin-4-carboxycilic acid 3 (0.133 g, 1 mmol) and trans-β-nitrostyrene 4a (0.149 g, 1 mmol) was heated under reflux for about 5 h (the progress of the reaction was monitored by TLC). After completion, the reaction mixture was filtered and the precipitated solid was washed with 3 mL cold ethanol (70%) to afford pure product 5a–n.General information and apparatus
Melting points were measured on an Electrothermal 9100 apparatus. NMR spectra were recorded with a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H, 100.6 MHz for 13C) with CDCl3 as solvent. IR spectra were recorded on an FT-IR Bruker vector 22 spectrometer. Mass spectra were recorded on a Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses were carried with a Perkin-Elmer 2400II CHNS/O Elemental Analyzer.Acknowledgements
The authors acknowledge the University of Mazandaran for financial support of this research.Notes and references
- (a) J. W. Lown, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, NY, 1984, vol. 1, p. 2 Search PubMed; (b) C. Najera and J. M. Sansano, Angew. Chem., 2005, 117, 6428 CrossRef PubMed; (c) K. V. Gothelf, in Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, 2002, pp. 211–247 Search PubMed; (d) K. V. Gothelf and K. A. Jørgensen, Chem. Rev., 1998, 98, 863 CrossRef CAS PubMed; (e) N. V. Lakshmi, P. Thirumuruga and P. T. Perumal, Tetrahedron Lett., 2010, 51, 1064 CrossRef CAS PubMed; (f) M. Poornachandran and R. Raghunathan, Synth. Commun., 2007, 37, 2507 CrossRef CAS PubMed.
- R. Grigg and S. Thianpatanagul, J. Chem. Soc., Chem. Commun., 1984, 180 RSC.
- (a) S. Indumathi, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 4978 CrossRef CAS PubMed; (b) R. S. Kumar, S. Perumal, K. A. Shetty, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 124 CrossRef CAS PubMed; (c) R. S. Kumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2010, 45, 411 CrossRef CAS PubMed; (d) K. Balamurugan, S. Perumal, A. S. K. Reddy, P. Yogeeswari and D. Sriram, Tetrahedron Lett., 2009, 50, 6191 CrossRef CAS PubMed; (e) S. V. Karthikeyan, S. Perumal, K. A. Shetty, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2009, 19, 3006 CrossRef CAS PubMed; (f) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 3821 CrossRef PubMed; (g) R. R. Kumar, S. Perumal, S. C. Manju, P. Bhatt, P. Yogeeswari and D. Sriram, Bioorg. Med. Chem. Lett., 2009, 19, 3461 CrossRef PubMed; (h) T. Okita and M. Isobe, Tetrahedron, 1994, 50, 11143 CrossRef CAS; (i) P. Rosenmond, M. Hosseini-Merescht and C. Bub, Liebigs Ann. Chem., 1994, 2, 151 CrossRef PubMed; (j) M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892 CrossRef CAS.
- M. Hasegawa, A. Nakayama, S. Yokohama, T. Hosokami, Y. Kurebayashi, T. Ikeda, Y. Shimoto, S. Ide, Y. Honda and N. Suzuki, Chem. Pharm. Bull., 1995, 43, 1125 CrossRef CAS.
- (a) J. E. Baldwin, R. T. Freeman, C. Lowe, C. J. Schofield and E. Lee, Tetrahedron Lett., 1989, 45, 4537 CrossRef CAS; (b) J. E. Baldwin, C. Lowe, C. J. Schofield and E. Lee, Tetrahedron Lett., 1986, 27, 3461 CrossRef CAS.
- (a) T. D. Aicher, D. C. Knorr and H. C. Smith, Tetrahedron Lett., 1998, 39, 8579 CrossRef CAS; (b) T. D. Aicher, B. Balkan, P. A. Bell, L. J. Brand, S. H. Cheon, R. O. Deems, J. B. Fell, W. S. Fillers, J. D. Fraser, J. Gao, D. C. Knorr, G. G. Kahle, C. L. Leone, J. Nadelsen, R. Simpson and H. C. Smith, J. Med. Chem., 1998, 41, 4556 CrossRef CAS PubMed.
- (a) G. Trapani, M. Franco, A. Latrofa, G. Genchi, M. Brigiani, M. Mazzoccoli, M. Persichel-la, M. Serra, G. Biggio and G. Liso, Eur. J. Med. Chem., 1994, 29, 197 CrossRef CAS; (b) G. Trapani, M. Franco, A. Latrofa, A. Carotti, S. Cellamare, M. Serra, C. A. Ghiani, G. Tuligi, G. Biggio and G. Liso, J. Pharm. Pharmacol., 1996, 48, 834 CrossRef CAS PubMed.
- (a) R. J. Monlineux, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1987, ch. 1 Search PubMed; (b) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS PubMed; (c) C. V. Gazlliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef PubMed; (d) B. M. Trost and M. K. Brennan, Synthesis, 2009, 3003 CrossRef CAS; (e) S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS; (f) F. Zhou, Y. L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS PubMed.
- G. Wu, L. Ouyang, J. Liu, S. Zeng, W. Huang, B. Han, F. Wu, G. He and M. Xiang, Mol. Diversity, 2013, 17, 271 CrossRef CAS PubMed; S. Haddad, S. Boudriga, F. Porzio, A. Soldera, M. Askri, D. Sriram, P. Yogeeswari, M. Knorr, Y. Rousselin and M. M. Kubicki, RSC Adv., 2014, 4, 59462 RSC.
- K. Atul, M. R. Awatar, S. A. Kumar, S. A. Bahadur and T. A. Kumar, Indian Pat. Appl., 2011, 48, 20110114 Search PubMed.
- A. Kumar, G. Gupta, S. Srivastava, A. K. Bishnoi, R. Saxena, R. Kant, R. S. Khanna, P. R. Maulikand and A. Dwivedi, RSC Adv., 2013, 3, 4731 RSC.
- S. Sivakumar, R. R. Kumar, M. A. Ali and T. S. Choon, Eur. J. Med. Chem., 2013, 65, 240 CrossRef CAS PubMed.
- A. Kamal, K. L. Reddy, V. Devaiah, N. Shankaraiah and M. V. Rao, Mini-Rev. Med. Chem., 2006, 6, 71 CrossRef CAS.
- (a) A. Gazit, H. App, G. McMohan, J. Chen, A. Levitzki and F. D. Bohmer, J. Med. Chem., 1996, 39, 2170 CrossRef CAS PubMed; (b) U. Sehlstedt, P. Aich, J. Bergman, H. Vallberg, B. Nordon and A. Graslund, J. Mol. Biol., 1998, 278, 31 CrossRef CAS PubMed.
- (a) Y. Sarrafi, M. Sadatshahabi, M. Hamzehloueian, K. Alimohammadi and M. Tajbakhsh, Synthesis, 2013, 45, 2294 CrossRef CAS; (b) K. Alimohammadi, Y. Sarrafi, M. Tajbakhsh, S. Yeganegi and M. Hamzehloueian, Tetrahedron, 2011, 67, 1589 CrossRef CAS PubMed; (c) Y. Sarrafi, M. Hamzehlouian, K. Alimohammadi and H. R. Khavasi, Tetrahedron Lett., 2010, 51, 4734 CrossRef CAS PubMed; (d) Y. Sarrafi, M. Hamzehloueian, K. Alimohammadi and S. Yeganegi, J. Mol. Struct., 2012, 1030, 168 CrossRef CAS PubMed; (e) M. Hamzehloueian, S. Yeganegi, Y. Sarrafi, K. Alimohammadi and M. Sadatshahabi, J. Serb. Chem. Soc., 2014, 79, 1 CrossRef.
- ESI†.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven Jr, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, Z. A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- (a) A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735 CrossRef CAS PubMed; (b) A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899 CrossRef CAS.
- P. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. V. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317 CrossRef CAS.
- M. K. Cyranski, T. M. Krygowski, A. R. Katritzky and P. V. R. Schleyer, J. Org. Chem., 2002, 67, 1333 CrossRef CAS PubMed.
- K. Wolinski, J. F. Hilton and P. Pulay, J. Am. Chem. Soc., 1990, 112, 8251 CrossRef CAS.
- (a) T. Schwabe and S. Grimme, Acc. Chem. Res., 2008, 41, 569 CrossRef CAS PubMed; (b) M. Korth and S. Grimme, J. Chem. Theory Comput., 2009, 5, 993 CrossRef CAS.
- (a) Y. Hagiwara and M. Tateno, J. Phys.: Condens. Matter, 2009, 21, 245103 CrossRef PubMed; (b) B. J. Lynch, P. L. Fast, M. Harris and D. G. Truhlar, J. Phys. Chem., 2000, 104, 4811 CrossRef CAS; (c) Y. Zhao, O. Tishchenko and D. G. Truhlar, J. Phys. Chem., 2005, 109, 19046 CrossRef CAS PubMed; (d) R. Peverati and K. K. Baldridge, J. Chem. Theory Comput., 2008, 4, 2030 CrossRef CAS; (e) S. Tsuzuki and H. P. Luthi, J. Chem. Phys., 2001, 114, 3949 CrossRef CAS PubMed; (f) J. A. Duncan and M. C. Spong, J. Phys. Org. Chem., 2005, 18, 462 CrossRef CAS PubMed; (g) P. C. Redfern, P. Zapol, L. A. Curtiss and K. Raghavachari, J. Phys. Chem., 2000, 104, 5850 CrossRef CAS.
- (a) J. Antony and S. Grimme, Phys. Chem. Chem. Phys., 2006, 8, 5287 RSC; (b) S. Grimme, J. Antony, T. Schwabe and C. Muck-Lichtenfeld, Org. Biomol. Chem., 2007, 5, 741 RSC; (c) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 19 CrossRef PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS.
- (a) C. C. Sobhi, A. K. Nacereddine, A. Djerourou, M. J. Aurell and L. R. Domingo, Tetrahedron, 2012, 68, 8457 CrossRef CAS PubMed; (b) Y. Du, E. H. Krenske, J. E. Antoline, A. G. Lohse, K. N. Houk and R. P. Hsung, J. Org. Chem., 2013, 78, 1753 CrossRef CAS PubMed; (c) A. Dieckmann and K. N. Houk, J. Chem. Theory Comput., 2012, 8, 5064 CrossRef CAS; (d) E. H. Krenske, S. He, J. Huang, Y. Du, K. N. Houk and R. P. Hsung, J. Am. Chem. Soc., 2013, 135, 5242 CrossRef CAS PubMed; (e) P. H. Y. Cheong, C. Y. Legault, J. M. Um, N. Çelebi-ölçüm and K. N. Houk, Chem. Rev., 2011, 111, 5042 CrossRef CAS PubMed; (f) A. Lumbroso, S. Catak, S. Sulzer-Mossé and A. de Mesmaeker, Tetrahedron Lett., 2014, 55, 5147 CrossRef CAS PubMed; (g) X. Xu, P. Liu, X. Shu, W. Tang and K. N. Houk, J. Am. Chem. Soc., 2013, 135, 9271 CrossRef CAS PubMed.
- Y. Lan, L. Zou, Y. Cao and K. N. Houk, J. Phys. Chem., 2011, 115, 13906 CrossRef CAS PubMed.
- (a) W. T. Borden, R. J. Loncharich and K. N. Houk, Annu. Rev. Phys. Chem., 1988, 39, 213 CrossRef CAS; (b) A. Moyano, M. A. Pericàs and E. Valentí, J. Org. Chem., 1989, 54, 573 CrossRef CAS; (c) F. P. Cossío, I. Morao, H. Jiao and P. V. R. Schleyer, J. Am. Chem. Soc., 1999, 121, 6737 CrossRef; (d) I. Morao, B. Lecea and F. P. Cossío, J. Org. Chem., 1997, 62, 7033 CrossRef; (e) B. Lecea, A. Arrieta, G. Roa, J. M. Ugalde and F. P. Cossío, J. Am. Chem. Soc., 1994, 116, 9613 CrossRef CAS; (f) B. Lecea, A. Arrieta, X. Lopez, J. M. Ugalde and F. P. Cossío, J. Am. Chem. Soc., 1995, 117, 12314 CrossRef CAS; (g) A. Arrieta, D. Otaegui, A. Zubia, F. P. Cossio, A. Diaz-Ortiz, A. Hoz, M. A. Herrero, P. Prieto, C. F. Foces, J. L. Pizarro and M. I. Arriortua, J. Org. Chem., 2007, 72, 4313 CrossRef CAS PubMed.
- K. B. Wiberg, Tetrahedron, 1968, 24, 1083 CrossRef CAS.
- K. N. Houk, Acc. Chem. Res., 1975, 8, 361 CrossRef CAS.
- (a) P. Rooshenas, K. Hof, P. R. Schreiner and C. M. Williams, Eur. J. Org. Chem., 2011, 983 CrossRef CAS PubMed; (b) R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512 CrossRef CAS; (c) R. G. Parr and W. Yang, in Density Functional Theory of Atoms and Molecules, Oxford University, NewYork, 1989 Search PubMed.
- (a) M. Rahm and T. Brinck, J. Phys. Chem. A, 2008, 112, 2456 CrossRef CAS PubMed; (b) K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 1998, 98, 863 CrossRef CAS PubMed.
- R. G. Parr, L. V. Szentpaly and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922 CrossRef CAS.
- (a) L. R. Domingo, M. J. Aurell, P. Perez and R. Contreras, Tetrahedron, 2002, 58, 4417 CrossRef CAS; (b) L. R. Domingo, A. Asensio and P. Arroyo, J. Phys. Org. Chem., 2002, 15, 660 CrossRef CAS PubMed; (c) L. R. Domingo, M. Arno, R. Contreras and P. Perez, J. Phys. Chem. A, 2002, 106, 952 CrossRef CAS; (d) L. R. Domingo, Tetrahedron, 2002, 58, 3765 CrossRef CAS; (e) L. R. Domingo and M. J. Aurell, J. Org. Chem., 2002, 67, 959 CrossRef CAS PubMed; (f) L. R. Domingo, M. J. Aurell, P. Perez and R. Contreras, J. Org. Chem., 2003, 68, 3884 CrossRef CAS PubMed; (g) L. R. Domingo and J. Andres, J. Org. Chem., 2003, 68, 8662 CrossRef CAS PubMed.
- L. R. Domingo, M. J. Aurell, P. Perez and R. Contreras, J. Phys. Chem. A, 2002, 106, 6871 CrossRef CAS.
- (a) K. Fukui, Acc. Chem. Res., 1981, 14, 363 CrossRef CAS; (b) K. Fukui, Acc. Chem. Res., 1971, 4, 57 CrossRef CAS; (c) K. Fukui, in Molecular Orbitals in Chemistry, Physics, and Biology, Academic, New York, NY, 1964, p. 525 Search PubMed.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- (a) A. K. Chandra and M. T. Nguyen, J. Phys. Chem., 1998, 102, 6181 CrossRef CAS; (b) T. L. Nguyen, F. de Proft, A. K. Chandra, T. Uchimaru, M. T. Nguyen and P. Geerlings, J. Org. Chem., 2001, 66, 6096 CrossRef PubMed; (c) D. Sengupta, A. K. Chandra and M. T. Nguyen, J. Org. Chem., 1997, 62, 6404 CrossRef CAS; (d) S. Damoun, G. van de Woude, F. Mendez and P. Geerlings, J. Phys. Chem., 1997, 101, 886 CrossRef CAS; (e) A. K. Chandra and M. T. Nguyen, Int. J. Mol. Sci., 2002, 3, 310 CrossRef CAS PubMed.
- H. Chermette, J. Comput. Chem., 1999, 20, 129 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1409005. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra14071d |
| This journal is © The Royal Society of Chemistry 2015 |





