A facile route to prepare few-layer graphene/polyamide 6 nanocomposites by liquid reactive extrusion†
Abstract
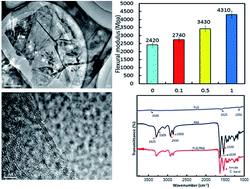
In this work, a liquid reactive extrusion process was developed to prepare few-layer graphene (FLG)/polyamide 6 (PA6) nanocomposites with low loading of nanofillers. Mass graphene flakes were fabricated by a re-expansion and exfoliation approach. The structure and morphology of FLG and FLG/PA6 nanocomposites were characterized by Raman spectroscopy, X-ray photoelectron spectroscopy, high-resolution transmission electron microscopy, field-emission scanning electron microscopy and Fourier-transformed infrared analysis. Moreover, the mechanical and crystallization properties of the PA6 and FLG/PA6 nanocomposites were also observed.

 Please wait while we load your content...
Please wait while we load your content...