Multifunctional core–shell Co–SiO2 nanowires via electrodeposition and sol–gel techniques
Abstract
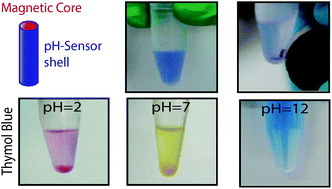
In this work, we propose a new strategy for the synthesis of multifunctional nanowires using a combination of sol–gel and electrodeposition techniques, based on a two-step procedure. First of all, nanotubes of SiO2 are synthesized via a sol–gel technique using polycarbonate membranes as templates. Homogenous nanotubes are obtained after centrifugation and thermal annealing. Afterwards, a ferromagnetic cobalt core is grown using potentiostatic electrodeposition. Finally, the core–shell Co–SiO2 nanowires are released by dissolving the template using wet-etching. These nanodevices can be used for many detection and sensing purposes. As a proof of concept, we have developed a pH nanosensor by including a pH-sensitive organic dye in the SiO2 shell. The sensing principle is based on the optical response of the organic dye towards pH when added to a solution. The magnetic core allows the recovery of the nanosensors after use. These nanowires can therefore be used as recoverable pH nanosensors. By changing the dye molecule to another molecule or receptor, the procedure described in the paper can be used to synthesize nanodevices for many different applications.

 Please wait while we load your content...
Please wait while we load your content...