The enhanced photocatalytic properties of BiOCl/BiVO4 p–n heterojunctions via plasmon resonance of metal Bi†
Abstract
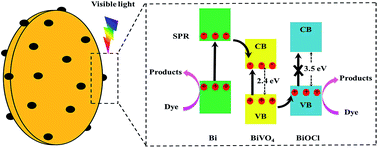
Novel p–n heterojunctions of BiOCl/BiVO4 nanosheets have been first fabricated via a one step method, which becomes a unified integration due to the substation process and exhibits higher photocatalytic performance than each pure component. Moreover, the photocatalytic and photoelectrochemical activities of BiOCl/BiVO4 hybrids can be further improved by depositing metallic Bi owing to its plasmon resonance. Metallic Bi can not only furnish extra electrons to enhance the photocurrent but also supply an oxidation position to degrade organic contaminants. In addition, although BiOCl could not be excited by visible light, it could capture holes from the valance band of BiVO4, which would effectively facilitate the separation of photogenerated electron–hole pairs and thus significantly improve the photocatalytic properties of BiVO4.

 Please wait while we load your content...
Please wait while we load your content...