Facile synthesis of oleylamine-capped silver nanowires and their application in transparent conductive electrodes†
Abstract
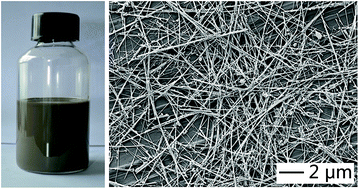
We report a facile method for the synthesis of oleylamine-capped silver (Ag) nanowires in high purity. For the first time, Ag nanowires could be produced in high purity via a simple one-pot approach in a hydrophobic phase. The success of this synthesis relies on the use of Cu2+ to mediate the reduction of silver bromide (AgBr) by oleylamine at an elevated temperature, which promoted the high-yield formation of Ag products with a wire-like shape. These Ag nanowires were washed and deposited on PET films to form a transparent conductive electrode (TCE), which showed a sheet resistance of 34.0 Ω sq−1 and an optical transmittance of 70–80% at visible wavelengths. In addition to TCEs, these nanowires could also find important applications in the fields of conductive ink, and wearable electronics, among others.

 Please wait while we load your content...
Please wait while we load your content...