Solution properties and phase behavior of a combination flooding system consisting of hydrophobically amphoteric polyacrylamide, alkyl polyglycoside and n-alcohol at high salinities†
Rui Liuab,
Wanfen Pu*ab,
Lili Wangc,
Quansheng Chend,
Zhihong Lid,
Yu Lie and
Bin Lib
aState Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu, Sichuan 610500, China. E-mail: propwf58@163.com; Tel: +86 15928967990
bPetroleum Engineering Institute, Southwest Petroleum University, Chengdu, Sichuan 610500, China
cChangqing Oilfield Company Third Gas Production Plant, Inner Mongolia 017300, China
dExperimental Detection Research Institute, Xinjiang Oilfield Branch Company, PetroChina, Karamay, Xinjiang 834000, China
eCNOOC Energy Technology-Drilling & Production Co., Tianjin 300458, China
First published on 3rd August 2015
Abstract
Application of polymer/surfactant (SP) combination flooding technique is attracting considerable interest in enhanced oil recovery (EOR). In this study, a SP combination system consisting of hydrophobically amphoteric polyacrylamide (HMPAM), C12/14 alkyl polyglycoside (APG1214), and n-alcohol was designed, and its comprehensive performance, including viscosity, oil/water interfacial tension (IFT) and phase behavior was systematically and thoroughly investigated. Four types of dehydrated crude oils with different hydrocarbon component distributions were employed in the experiments. The intermolecular interactions, which were induced by hydrophobic moieties of HMPAM, endowed this unique polymer with favorable thickening efficiency under harsh reservoir conditions. APG1214, prepared from renewable raw materials, reached low IFT within a wide salinity region. In addition, the coadsorption of HMPAM and APG1214 at the crude oil/water interface to form polymer–surfactant complexes, which exhibited higher activity on reducing IFT, and the ultralow IFT were observed with addition of n-alcohol. The variation in zeta potential measurement indicated that HMPAM, APG1214 and n-alcohol cooperatively encircled oil droplets to manipulate the o/w emulsion. Moreover, emulsion phase behavior and particle size distribution analysis confirmed that the rigid cross-link network at the surface of the emulsion, which was constructed synergistically by HMPAM, APG1214 and n-alcohol, stabilized the emulsion phase.
1. Introduction
After water flooding, a large amount of original oil in place (OOIP) is capillary trapped as residual oil,1,2 which is the target for enhanced oil recovery (EOR). A series of systematic studies revealed that it is of importance to control the capillary number (Nc) to displace the occluded oil from the pores and capillaries of reservoir rock.3–6 Nc is a dimensionless quantity that can be well represented by the following equation:
 | (1) |
Recent advancement and improvement in laboratory investigations and field applications have led to an increased demand for polymer/surfactant (SP) combination flooding that can fulfill specific functions to improve oil recovery.4,7–10 The primary oil recovery mechanisms of SP combination flooding are summarized as follows: (1) increasing the viscosity of the injected water to maintain a favorable mobility ratio (M ≤ 1),9,11,12 (2) reducing the IFT between the oil and water phases,12,13 and (3) generating emulsion to extract residual oil from mature oil reservoirs.10,14 Moreover, SP overcomes the drawbacks of conventional alkali/polymer/surfactant (ASP) combination flooding technology, which involves reaction with divalent ions causing blockage of pipe, damage to equipment and impairment of reservoir formation due to the addition of alkali.9,14
However, with EOR strategy moving towards deeper, and consequently, hotter reservoirs, the screening of the SP flooding system to satisfy the harsh conditions of the reservoirs remains a considerable challenge. On the one hand, partially hydrolyzed polyacrylamide (HPAM), which is widely used as a mobility agent in EOR applications, is highly sensitive to shear and thermal degradation.15,16 In a salt solution, cationic ions shield the negative charges carried by carboxyl groups and produce all macromolecules with flexible chain shrinking, resulting in a dramatic decrease in viscosity.17 It is extensively reported that hydrophobically modified partially hydrolyzed polyacrylamides exhibit unique rheological behavior and emulsifying abilities.17–20 Above a certain polymer concentration, hydrophobic moieties associate and build transitory three dimensional networks, leading to excellent shear stability,21 good thermal endurance22 and salt resistance.16 This type of polymer can adsorb at the surface of oil droplets and provide a thick viscoelastic membrane to slow down droplet diffusion and coalescence.23–25 On the other hand, highly efficient surfactants, used to manipulate the IFT, modify wetting properties and reduce the capillary force to facilitate oil mobilization.26–29 Commonly employed surfactants in the petroleum industry are alkyl sulfonates and heavy alkyl benzene sulfonates.30,31 Although they are industrially feasible and compatible with crude oil, their properties are considerably temperature and salinity dependent. Temperature and salinity variations due to the geological circumstances of reservoirs are expected to reduce control of surfactant flooding, directly resulting in economic uncertainty.10 Recently, the use of alkyl polyglycosides prepared from renewable raw materials for EOR applications has attracted considerable attention due to their commercial feasibility, good biodegradability and eco-friendly characteristics.32,33 Most importantly, this novel surfactant has good performance on water/oil IFT and a phase behavior that is largely independent of temperature and salinity.29,34,35
Oil in water (o/w) emulsion indicates an ultralow IFT,36,37 and the phase behavior of surfactant/groundwater (brine)/oil systems is crucial for EOR due to the well-established relationship between the IFT and emulsion phase behavior.38,39 In addition, many studies have provided meaningful insight into the synergistic effects of polymer and surfactant formation on the stabilization of emulsion.6,23–25,40,41 Nevertheless, most of these investigations are focused on the properties of SP systems that are based on model oil, and therefore have some discrepancies compared to oil reservoir conditions because the composition of crude oil is more complicated.
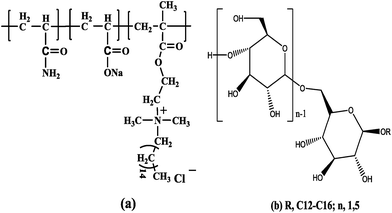
The aim of this study was to construct a SP combination system that had ideal properties at high salinities for EOR. A long-chain alkyl cationic surfmer, methacryloxyethyl-dimethyl cetyl ammonium chloride (DMDCC), modified amphoteric polyacrylamide (hereafter referred to as HMPAM) (Fig. 1a) was preferentially synthesized.42 C12/14 alkyl polyglycoside (hereafter referred to as APG1214) (Fig. 1b) was selected as the surfactant and n-alcohol was utilized as a co-solvent because it plays a significant role as a physical linker between the oleic phase and the APG1214 monolayer to tune the hydrophilic–lipophilic balance (HLB) of APG1214.10,35 The water/oil IFT as well as the phase behavior of the polymer/APG1214/n-alcohol formulations (varying the HMPAM concentration, APG1214 concentration, and n-alcohol type and concentration) and four dehydrated crude oils were systematically investigated. Zeta (ζ) potential measurement was used to determine the role of HMPAM composition on the emulsion, and laser light scattering was employed to monitor the droplet size of a typical emulsion. Moreover, elements influencing the manipulation of emulsion, including HMPAM, APG1214 and n-alcohol, were interpreted. Finally, the appropriate formulations of SP combination systems based on reservoir conditions and the properties of crude oils were proposed.
2. Experimental section
2.1. Reagents and materials
Acrylamide (AM) purchased from Chengdu KeLong Chemical Reagent Co., Ltd (China) was recrystallized twice from acetone and vacuum dried at room temperature prior to use. A long-chain alkyl cationic surfmer, methacryloxyethyl-dimethyl cetyl ammonium chloride (DMDCC), was synthesized according to a method reported elsewhere.42 The azo-initiator V-50 (2,2′-azobis (2-amidinopropane) dihydrochloride) (UA, Alfa Aesar 99%) was used without further purification. APG1214, a commercial product, was supplied by Shanghai Fine Chemical Co., Ltd (China). All the ESI† were obtained from Aldrich (analytically pure) and used as received. Water was double deionized with a Millipore Milli-Q system to produce 18 MΩ deionized water.Four different dehydrated crude oils were obtained from Huabei oilfield, Dagang oilfield, Changqing oilfield and Tahe oilfield. Synthetic water injected in different reservoirs used in the study was prepared in accordance with the inorganic composition of formation water (see Table 1). The hydrocarbon component distribution of the four dehydrated oils is depicted in Table 2.
| Items | Huabei oilfield | Dagang oilfield | Changqing oilfield | Tahe oilfield |
|---|---|---|---|---|
| K+ and Na+ (mg l−1) | 4008.8 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 807.4 807.4 |
1094.03 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 876.4 876.4 |
| Ca2+and Mg2+ (mg l−1) | 263 | 109.3 | 201.42 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889 889 |
| Cl− (mg l−1) | 4697.8 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665.9 665.9 |
408.78 | 7411.2 |
| SO42− (mg l−1) | 361.4 | 325.3 | 2501.87 | 441.8 |
| HCO3− (mg l−1) | 2308 | 5414.2 | 2256.3 | 487.2 |
| Total salt (mg l−1) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639 639 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322 322 |
6495 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106 |
| pH | 6.8 | 6.5 | 7.2 | 7.6 |
| Temperature °C | 70 | 67 | 68 | 65 |
| Apparent viscosity (mPa s) | 20.6 | 14.7 | 3.2 | 8.4 |
| Oil type | Huabei oilfield | Dagang oilfield | Changqing oilfield | Tahe oilfield | |
|---|---|---|---|---|---|
| Hydrocarbon component distribution (%) | |||||
| C2–C5 | 0.36 | 0.33 | 2.02 | 4.16 | |
| C6–C10 | 27.86 | 16.54 | 82.18 | 37.32 | |
| C11–C15 | 25.85 | 24.94 | 8.78 | 29.92 | |
| C16–C20 | 13.75 | 20.36 | 4.08 | 18.05 | |
| C21–C25 | 26.87 | 17.47 | 2.21 | 7.26 | |
| C26–C30 | 4.09 | 14.76 | 0.38 | 2.95 | |
| C30+ | 1.19 | 5.59 | 0.35 | 0.33 | |
| API˙ | 26 | 36 | 46 | 42 | |
2.2. Synthesis of hydrophobically amphoteric polyacrylamide
The detailed synthesis of HMPAM and HPAM is provided in the ESI.† The IR spectrum and 1H NMR spectra of typical HMPAM were obtained and are shown in Fig. S1 in the ESI.†2.3. Surface activity of APG1214
The method for the measurement of the surface properties of APG1214 is documented in the ESI.†2.4. Measurement of viscosity
The apparent viscosity of solution samples was determined by a Brookfield DV-III rheometer equipped with different sizes of spindles at a shear rate of 7.34 s−1.2.5. Oil/water interfacial tension and phase behavior
The equilibrium IFT between crude oil and solution samples was measured using a TX-500 spinning drop tensiometer (KINO Industry Co. Ltd, USA) at a rotational speed of 7000 rpm for 30 min under the subterranean strata temperature. Test tube samples were prepared with 14 mL of SP solution and 6 mL of dehydrated crude oil. The tube samples were mixed well using an OWC-4060 homogenizer (Delixi Group Co. Ltd, China) at a rotating speed of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. They were allowed to stand for 24 h to allow the fluids to come to phase equilibrium, then the relative volumes of the oil, aqueous and emulsion phases of the samples were recorded.
000 rpm for 10 min. They were allowed to stand for 24 h to allow the fluids to come to phase equilibrium, then the relative volumes of the oil, aqueous and emulsion phases of the samples were recorded.
2.6. Hydrodynamic diameter of the SP combination system
The hydrodynamic diameters of the samples were measured using a BI-200SM wide angle dynamic laser light scattering instrument (DLS).2.7. ζ potential and particle size distribution of emulsion
ζ potential and droplet size distribution measurements of the emulsion were conducted using a hybrid Malvern Zetasizer (Malvern Instruments Ltd, Worces-tershire, UK). The purpose of ζ potential measurement is to determine the composition of HMPAM on the formation of emulsion; thus, the samples were prepared with deionized water. The morphologies of the emulsion were observed by an environment scanning electron microscope (Philips SEM XL 30).3. Results and discussion
3.1. Mobility control capacity of HMPAM
Viscosification is necessary for a polymer employed in EOR.43 In the ultra-dilute solution regime, the viscosity of HMPAM was lower than that of HPAM due to intramolecular association, leading to the coiling of chains (see Fig. 2). Above a critical concentration (CAC, 750 mg l−1), an upward curvature in the viscosity for HMPAM could be observed due to the building of intermolecular association of hydrophobic moieties by the transitory three-dimensional networks. Salinity significantly affected the apparent viscosity of polymer solutions (see Fig. 3). For HPAM, the decreased viscosity was observed because cationic ions shielded the negative charges carried by carboxyl groups (–COO−), resulting in macromolecular flexible chain sharking.15 The apparent viscosity for HPAM solution was just 20% of the original value in deionized water at the highest salt concentration. The apparent viscosity for the HMPAM solution first decreased dramatically with a small amount of salt; this was interpreted because the macromolecular chain of HMPAM was partly compacted by the presence of cationic ions, especially divalent cations (Ca2+, Mg2+).44 With increasing salt concentration, the intermolecular association becomes favored by the addition of electrolytes,11 and salt-thickening behavior was observed. Upon further increasing the salt concentration, the hydrophobic microstructures turned to compacted and isolated macromolecules, resulting in the lower degree of intermolecular interaction. Notably, the viscosity of HMPAM at 70 °C was considerably lower than that at ambient temperatures. However, even at the highest salt concentration, it was higher than that for HPAM, indicating that the incorporation of a small amount of hydrophobic groups into the main backbones improved the salt resistance for HMPAM. | ||
| Fig. 2 Zero shearing viscosity for HMPAM and HPAM samples as a function of polymer concentration (T = 25 °C). | ||
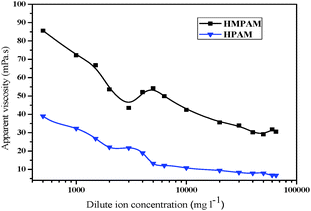 | ||
| Fig. 3 Apparent viscosity of HMPAM and HPAM as a function of dilute ion concentration (polymer concentration of 1250 mg l−1, Tahe oilfield injected water, T = 70 °C). | ||
3.2. Screening of APG1214 concentration
APG1214 shows good brine compatibility, and the IFT and emulsion consisting of APG1214, oil and water are indifferent to temperature and salinity within a wide salt region.10,26,29 The equilibrium IFT of dehydrated crude oil and APG1214 solution as a function of surfactant concentration is presented in Fig. 4. We observed low IFT (10−1 to 10−2 Nm m−1), but not ultralow IFT (less than 10−2 Nm m−1), this phenomenon is investigated in the following study.3.3. Viscosity and IFT of the SP combination system under simulated reservoir conditions
For the HMPAM/APG1214 solution, surfactant molecules can serve as bridge linkers between hydrophobic moieties of polymer chains; thus, vastly alter the dynamic rheology of the mixed solutions.34,35Generally, the viscosity increased and exhibited a maximum value at a small amount of APG1214, and then dropped to a plateau region with a further increase in the concentration of the surfactant (Fig. 5). According to the hypothesis established by McCormick et al.,45 hydrophobic interactions occur with hydrophobic moieties of the HMPAM backbone with the alkyl chain of APG1214, resulting in mixed micelle formation; these micelles serve as junction points for supermolecular network formation, leading to pronounced increases in the apparent solution viscosity. Beyond the critical micelle concentration (CMC) of APG1214, micelle bridging was disrupted and viscosity decreased. The mixed surfactant composed of APG1214 and n-alcohol could lower the APG1214 concentration, and tune the SP solution properties.27 A small amount of 1-dodecanol could reasonably mediate the HMPAM/APG1214 solution viscosity in a wide APG1214 concentration regime and weaken the disadvantage of the surfactant effect. The fact that n-alcohol tuned the SP solution property was well explained by the hydrodynamic diameter distribution of the SP solution (Fig. 6). Above the CAC, intermolecular associations for HMPAM chains enhanced their hydrodynamic size (870.6 nm for the HMPAM solution). However, the hydrophobic moieties of HMPAM were solubilized into micelles that were constructed by the APG1214 molecules when the APG1214 concentration was well beyond the CMC, which impaired the intermolecular interactions, and thus decreased the hydrodynamic size (545.8 nm for HMPAM/APG1214 solution). With the addition of a small amount of 1-dodecanol, both the HLB of the APG1214 system and the associative characteristics of HMPAM were well adjusted by these small molecules, therefore mediating the hydrodynamic scale (686.7 nm).
A synergistic interaction between HMPAM and APG1214 was generated to obtain lower IFT in comparison to individual APG1214 (Fig. 7). The mechanism when the oil/water IFT was reduced involved the chemical potential of the surfactant molecule adsorbed at the oil/water interface being equal to that required for micelle formation. Above the CMC, excess surfactant was preferentially solubilized as micelles and the IFT reached a minimum.46 On addition of surface active HMPAM, the hydrophobic unit of HMPAM tended to be in contact with the oil drops, while the hydrophilic part was in the water phase. The co-arrangement of HMPAM and APG1214 at the crude oil/water interface formed the polymer–surfactant complexes PSr.40,46,47 These types of complex have higher activity for reducing the IFT than individual APG1214 because the supermolecular effect induced by hydrophobic interactions between the hydrophobic moieties of HMPAM and the alkylchain of APG1214 make the chemical potential favorable for the complexes PSr. This is consistent with other studies.48,49 Moreover, the SP combination system consisting of HMPAM, APG1214 and 1-dodecanol reached the ultralow IFT. This can be well explained by the fact that n-alcohol acts as a co-surfactant, filling in the space formed by APG1214 molecules (or HMPAM–APG1214 complexes) and changes the “wedge” angle of the interfacial layer (more close interfacial packing),10,13,35,37 thus leading to the ultralow IFT.
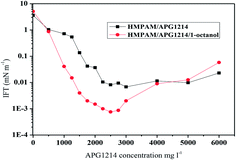 | ||
| Fig. 7 IFT of HMPAM/APG1214/1-dodecanol and HMPAM/APG1214 as a function of surfactant concentration (cHMPAM of 1250 mg l−1, c1-dodecanol of 800 mg l−1, Huabei crude oil and formation water, 70 °C). | ||
3.4. HMPAM modified properties of emulsion
The images in Fig. 8 depict the transition from unobserved to observed emulsions. As shown in Fig. 8a, no emulsion phase was present for the APG1214 concentration of 1500 mg l−1 and the HPAM concentration of 1000 mg l−1. The emulsion phase was observed when HPAM was replaced by HMPAM, but it was not obvious at the HMPAM concentration of 500 mg l−1. The emulsion volume was measured as a function of HMPAM concentration at a fixed APG1214 concentration of 1500 mg l−1 (Fig. 8b–d). The emulsion volume was not directly proportional to the HMPAM concentration, which remarkably increased with increasing HMPAM concentration from 500 to 1250 mg l−1, and slightly increased upon a further increase in HMPAM (1500 mg l−1). In the presence of 1-octanol, the widest emulsion region was observed (Fig. 8e–f).From this observation, we speculate that the hydrophobic moieties of HMPAM and APG1214 cooperatively govern the formation of emulsion. The ζ potential measurement of the emulsion was conducted to further confirm our hypothesis (Fig. 9). The ζ potential measurement was conducted above the concentration at which APG1214 free micelles formed in deionized water. At first, we tried to measure the ζ potential of the emulsion constructed by HPAM/APG1214. However, the unstable emulsion made it impossible to determine it. In the presence of 1-octanol, we measured the ζ potential of the emulsion, which remained the same with the HPAM concentration switching from 1250 to 1500 mg l−1.
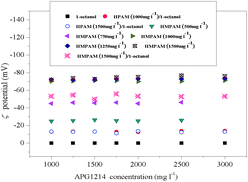 | ||
| Fig. 9 ζ values for SP solutions as a function of APG1214 concentration (c1-octanol of 800 mg l−1, Changqing crude oil, deionized oil). | ||
The variation in the emulsion ζ potential with APG1214 concentration at different HMPAM concentrations exhibited interesting properties, which deeply correlated with the regions of emulsion. For the APG1214/1-octanol mixed solution, we observed that the emulsion droplets were almost nonionic (neutrally charged). The ζ potential value was close to zero due to both APG1214 and 1-octanol being nonionic substances. For addition of 500 mg l−1 of HMPAM, a negative ζ potential value of −25.0 mV was observed. It is obvious that the large net negative charges of HMPAM led to the negative potential of the emulsion surface. The ζ potential value notably increased from −45.2 to −70.5 mV with increasing HMPAM concentration from 1000 to 1500 mg l−1, and it remained the same with further addition of HMPAM.
At the lowest HMPAM concentration of 500 mg l−1, polymer amount was insufficient to interact with APG1214 at the emulsion surface and therefore the lowest negative potential was exhibited. For both 1000 and 1500 mg L−1 concentrations of HMPAM, the ζ potential increased with increasing APG1214 concentration, but remained almost constant even at the highest APG1214 concentration. This implied that excessive APG1214 molecules and HMPAM macromolecules would not adsorb at the surface of the emulsion. The variation in the emulsion ζ potential confirmed the hypothesis that the supermolecular effect between the hydrophobic moieties of HMPAM and APG1214 hydrophobic tail induced HMPAM and APG1214 synergistic adsorption at the oil droplets surface to construct the o/w emulsion. Note that the ζ potential value of the emulsion in the presence of 1-octanol was slightly lower than that of emulsion in the absence of 1-octanol at the identical surfactant and polymer concentration. It is true that the adsorption of 1-octanol at the oil/water interface can partly reduce the space for HMPAM and APG1214.
3.5. Crude oil dominated n-alcohol chain length
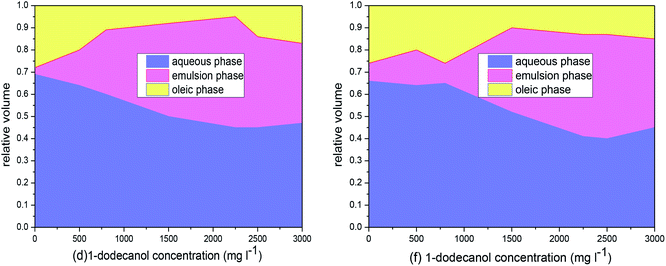
We also investigated the phase behavior and physicochemical properties of the emulsion phase for APG1214/n-alcohol/brine/crude oil and HMPAM/APG1214/n-alcohol/brine/crude oil systems, as a function of n-alcohol chain length (pentyl-, octyl-, and dodecyl-) and n-alcohol concentration (0, 500, 800, 1500, 2250, 2500 and 3000 mg l−1). The pseudoternary phase diagram has been constructed and shown in Fig. 10 and 11. In these figures, the aqueous phase consisting of brine, HMPAM, APG1214 and n-alcohol was considered as a single pseudo-component, crude oil was a single component (oleic phase) and the o/w emulsion was another single component (emulsion phase).For the SP combination system, emulsion phase behavior was closely related to the IFT but could not directly predict IFT (Fig. 7). Fundamentally, oil solubilization (emulsion phase) is a separate process that is controlled by the following mechanism:27,36 the oil solubilization capability of a surfactant is controlled by the overall free energy of the surfactant/brine/hydrocarbon system. The HLB of a surfactant is the main factor for determining the overall free energy. The emulsion phase was not observed by APG1214 individually. It was a consequence of the poor solubility of the APG1214 in the oleic phase over a wide salinity range. Emulsion was formed when the HLB of the APG1214 and the polarity of the oleic phase were well adjusted by adding an oil-soluble n-alcohol.
The emulsion phase was not observed for the individual APG1214, but it was observed with various amounts of n-alcohol added. For Changqing crude oil, the volume of emulsion increased and then reached a plateau with increasing amounts of 1-pentanol. A similar trend was observed for 1-octanol and 1-dodecanol. Moreover, the emulsion region was n-alcohol type dependent. The widest emulsion region was observed at the 1-octanol concentration of 2500 mg l−1, and the relative phase volumes for the aqueous phase, emulsion phase and oleic phase were 0.41, 0.4 and 0.19, respectively (Fig 10b). In the case of Tahe crude oil and Dagang crude oil, both 1-octanol and 1-dodecanol were good candidates for favorable emulsion (Fig. 10e–f). However, for the Huabei crude oil, 1-dodecanol would be the desired candidate in comparison to 1-pentanol and 1-octanol (Fig. 10l).
Through the analysis of the discrepancy of emulsion phase behavior versus the n-alcohol type, we speculate that crude oil dominates the chain length of n-alcohol. 1-Octanol was in good agreement with light hydrocarbon oil (Changqing crude oil). Both 1-octanol and 1-dodecanol would be compatible with medium hydrocarbon crude oil, for instance, Tahe crude oil and Dagang crude oil. The most favorable candidate would be 1-dodecanol in terms of weight hydrocarbon distribution of crude oil, for example Huabei crude oil. This appears to be contradictory to the “common review” that only 1-octanol is the best alcohol in terms of ultralow IFT and optimal emulsion phase for APG1214. It is true that these results are consistent with the literature.3,10,29,38 In the earlier studies, an n-alkane in the form of 1-octanol was utilized as a crude oil.35 1-Pentanol or 1-dodecanol was less effective in generating the emulsion phase, because the shorter alkyl chain for 1-pentanol was too soluble in the aqueous phase to play a physical linker between oil and aqueous solution, and the longer alkyl chain for 1-dodecanol was less likely to contact well with the light model oil. To summarise, an ideal n-alcohol, which would serve as a co-surfactant for desirable emulsion, must be compatible with the crude oil.
3.6. The mechanism of HMPAM/APG1214/n-alcohol synergistic stabilization of emulsion
 | ||
| Fig. 13 Representation of (a) APG1214/n-alcohol and (b) HMPAM/APG1214/n-alcohol at the emulsion surface. | ||
3.7. Formation of SP system
For cationic, ionic and zwitterionic surfactants, ultralow IFT and an emulsion phase were observed within a limited salinity range, which can be characterized by an optimal salinity.3,22,25,35 However, the salinity in the reservoir brine may vary in both areal and vertical zones due to years of water flooding, and limit the wide application of surfactant flooding or SP combination flooding for EOR. APG1214/n-alcohol and HMPAM/APG1214/n-alcohol formulations are largely indifferent to salinity within a wide salt region. Moreover, ultralow interfacial tensions were observed at the reservoir salt conditions (from 6495 mg l−1 of Changqing injected water to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 mg l−1 of Huabei injected water). Based on the comprehensive characters of HMPAM, APG1214 and n-alcohol, we managed to formulate SP systems that well satisfied reservoir conditions (temperature and salinity) and crude oil parameters (hydrocarbon distribution and viscosity of crude oil) from an effective point of view and is summarized in Table 3.
106 mg l−1 of Huabei injected water). Based on the comprehensive characters of HMPAM, APG1214 and n-alcohol, we managed to formulate SP systems that well satisfied reservoir conditions (temperature and salinity) and crude oil parameters (hydrocarbon distribution and viscosity of crude oil) from an effective point of view and is summarized in Table 3.
| Crude oil | Optimal SP systema | Properties | ||||
|---|---|---|---|---|---|---|
| HMPAM (mg l−1) | APG1214 (mg l−1) | n-Alcohol type and concentration (mg l−1) | IFT (mN m−1) | Viscosity (mPa s) | Phase behaviour (aqueous phase/emulsion phase/oleic phase)c | |
| a An optimal SP system was formulated at the corresponding temperature and salinity conditions.b The concentration of n-alcohol.c Relative volume of aqueous phase, emulsion phase and oleic phase, at the given n-alcohol amount. | ||||||
| Changqing crude oil | 1000 | 2500 | 1-Octanol (1500)b | 10−3 to 10−4 | 18.7 | 0.46/0.44/0.1 |
| Tahe crude oil | 1250 | 3000 | 1-Octanol (2250), 1-dodecanol (2250) | 10−3 to 10−4 | 22.3 | 0.42/0.46/0.12 (1-octanol), 0.38/0.49/0.13 (1-dodecanol) |
| Dagang crude oil | 1250 | 3000 | 1-Octanol (2250), 1-dedocanol (2250) | 10−2 to 10−3 | 28.7 | 0.46/0.42/0.12 (1-octanol), 0.41/0.46/0.13 (1-dodecanol) |
| Huabei crude oil | 1500 | 3250 | 1-Dedocanol (2500) | 10−2 to 10−3 | 43.3 | 0.35/0.55/0.1 |
4. Conclusions
1. In terms of mature oil reservoirs, the manipulation of Nc, including increase of viscosity of the injected liquid, reduction of the IFT between the oil and water phases, and, most importantly, formation of o/w emulsion (oil droplets covered by the injected liquid) is an effective strategy for EOR.2. The incorporation of a small amount of hydrophobic groups into the main backbones resulted in the hydrophobically amphoteric polyacrylamide (HMPAM), which had favorable thickening efficiency under harsh reservoir conditions.
3. APG1214 prepared from renewable raw materials could reach low IFT, which was largely independent of temperature and salinity. The coadsorption of HMPAM and APG1214 at the crude oil/water interface caused the formation of polymer–surfactant complexes that had higher activity for reducing IFT than a single APG1214. The SP combination system consisting of HMPAM, APG1214 and 1-alcohol reached the ultralow IFT because the “wedge” angle of the interfacial layer was adjusted by n-alcohol, which acted as a co-surfactant penetrated into the space formed by APG1214 molecules (or HMPAM–APG1214 complexes).
4. No emulsion phase was observed for an individual APG1214, whereas the supermolecular interaction between the hydrophobic moieties of HMPAM and APG1214 hydrophobic tail induced HMPAM and APG1214 adsorption at the oil droplets surface to construct the o/w emulsion.
5. Hydrocarbon component distribution for one crude oil dominated the optimum n-alcohol chain length.
6. Emulsion phase behavior, emulsion zeta potential measurement and particle size distribution analysis excellently indicated that HMPAM, APG1214 and n-alcohol coadsorbed at the surface of oil droplets to construct the rigid cross-link network and stabilized the emulsion phase.
7. Based on reservoir conditions and crude oil properties, SP systems consisting of HMPAM, APG1214 and n-alcohol were formulated. Moreover, other comprehensive properties for this novel system, such as the thermal stability, shear stability, adsorption behavior on reservoir rocks, the economic feasibility, and oil recovery efficiency, should be further investigated.
Acknowledgements
We are grateful to the Open Fund (PLN1417) by State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation (Southwest Petroleum University) and the Program to the Experimental Detection Research Institute, Xinjiang Oilfield Branch Company for the financial support to this study.Notes and references
- X. Z. Wang, W. L. Kang, X. C. Meng, M. Fan, J. W. Xu, J. B. Fu and Y. N. Zhang, Acta Phys.–Chim. Sin., 2012, 28, 2285–2290 CAS.
- C. Dai, Q. Ding, M. Zhao, J. Zhao, J. Fang and H. Li, RSC Adv., 2015, 5, 27978–27985 RSC.
- A. Bera, T. Kumar, K. Ojha and A. Mandal, Fuel, 2014, 121, 198–207 CrossRef CAS PubMed.
- S. Lee, D. H. Kim, C. Huh and G. A. Pope, Proceedings of the Society of Petroleum Engineers (SPE) Annual Technical Conference and Exhibition, New Orleans, Louisiana, 2009 Search PubMed.
- N. F. Najafabadi, M. Delshad, C. Han and K. Sepehrnoori, J. Pet. Sci. Eng., 2012, 86, 257–271 CrossRef PubMed.
- J. Hou, Z. Liu, S. Zhang and J. Yang, J. Pet. Sci. Eng., 2005, 47, 219–235 CrossRef CAS PubMed.
- H. Zhang, M. Dong and S. Zhao, Energy Fuels, 2010, 24, 1829–1836 CrossRef CAS.
- H. Zhang, M. Dong and S. Zhao, Energy Fuels, 2012, 26, 3644–3650 CrossRef CAS.
- Y. J. Guo, J. X. Liu, X. M. Zhang, R. S. Feng, H. B. Li, J. Zhang and P. Y. Luo, Energy Fuels, 2012, 26, 2116–2123 CrossRef CAS.
- S. Iglauer, Y. Wu, P. Shuler, Y. Tang and W. A. Goddard III, Colloids Surf., A, 2009, 339, 48 CrossRef CAS PubMed.
- A. Sabhapondit, A. Borthakur and I. Haque, Energy Fuels, 2003, 17, 683–688 CrossRef CAS.
- P. Shen, J. Wang, S. Yuan, T. Zhong and X. Jia, SPE J., 2009, 14, 237–244 CrossRef CAS.
- S. Iglauer, P. Wu, J. Shuler, M. Blanco, Y. Tang and W. A. Goddard III, Proceedings of the Society of Petroleum Engineers (SPE) Symposium on Improved Oil Recovery, Tulsa, Oklahoma, 2004 Search PubMed.
- K. Zhang and J. S. Qin, Pet. Sci. Technol., 2011, 29, 183–191 CrossRef CAS PubMed.
- R. S. Seright, A. Campbell, P. Mozley and P. Han, SPE J., 2010, 15, 341–348 CrossRef CAS.
- Y. Wu, A. Mahmoudkhani, P. Watson, T. Fenderson, and M. Nair, Proceedings of the Society of Petroleum Engineers (SPE) EOR Conference at Oil and Gas West Asia, Muscat, Oman, 2012 Search PubMed.
- L. Sun, W. Pu, J. Xin, P. Wei, B. Wang, Y. Li and C. Yuan, RSC Adv., 2015, 5, 23410–23418 RSC.
- C. Zhong, R. Huang, L. Ye and H. Dai, J. Appl. Polym. Sci., 2006, 101, 3996–4002 CrossRef CAS PubMed.
- B. Gao, H. Guo, J. Wang and Y. Zhang, Macromolecules, 2008, 41, 2890–2897 CrossRef CAS.
- W. Jaeger, J. Bohrisch and A. Laschewsky, Prog. Polym. Sci., 2010, 35, 511–577 CrossRef CAS PubMed.
- Y. Chang and C. L. McCormick, Macromolecules, 1993, 26, 6121–6126 CrossRef CAS.
- Q. Yang, C. Song, Q. Chen, P. Zhang and P. Wang, J. Polym. Sci., Part B: Polym. Phys., 2008, 46, 2465 CrossRef CAS PubMed.
- Q. Deng, H. Li, X. Cao, Y. Yang, X. Song and Y. Li, RSC Adv., 2015, 5, 13078–13086 RSC.
- W. Kang, B. Xu, Y. Wang, X. Shan, Y. Li, F. An and J. Liu, Colloids Surf., A, 2011, 384, 555–560 CrossRef CAS PubMed.
- I. M. Tucker, J. T. Petkov, C. Jones, J. Penfold, R. K. Thomas, S. E. Rogers and I. Grillo, Langmuir, 2012, 28, 14974–14982 CrossRef CAS PubMed.
- W. von Rybinsk and B. Guckenbiehl, Colloids Surf., A, 1998, 142, 333–342 CrossRef.
- B. Gao and M. M. Sharma, J. Colloid Interface Sci., 2013, 407, 375–381 CrossRef CAS PubMed.
- Y. Bayrak and M. Iscan, Colloids Surf., A, 2005, 268, 99–103 CrossRef CAS PubMed.
- M. J. Rosen and S. B. Sulthana, J. Colloid Interface Sci., 2001, 239, 528–534 CrossRef CAS PubMed.
- J. L. Cayais, R. S. Schechter and W. H. Wade, J. Colloid Interface Sci., 1977, 59, 31–38 CrossRef.
- P. H. Doe, W. H. Wade and R. S. Schechter, J. Colloid Interface Sci., 1977, 59, 525–531 CrossRef CAS.
- M. Salim, O. K. Abou-Zied, H. U. Kulathunga, A. Baskaran, U. R. Kuppusamy and R. Hashim, RSC Adv., 2015, 5, 55536–55543 RSC.
- P. Foley, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518 RSC.
- D. Obasi and B. Ghosh, Proceedings of the Society of Petroleum Engineers (SPE) Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2013 Search PubMed.
- S. Iglauer, Y. Wu, P. Shuler, Y. Tang and W. A. Goddard III, Tenside, Surfactants, Deterg., 2010, 47, 87–97 CrossRef CAS.
- M. Roshanfekr and R. T. Johns, Fluid Phase Equilib., 2011, 304, 52–60 CrossRef CAS PubMed.
- K. Fukuda, U. Olsson and M. Ueno, Colloids Surf., B, 2001, 20, 129 CrossRef CAS.
- J. J. Sheng, J. Pet. Sci. Eng., 2010, 75, 143–153 CrossRef CAS PubMed.
- Y. Zhu, G. Xu, H. Gong, D. Wu and Y. Wang, Colloids Surf., A, 2009, 332, 90–97 CrossRef CAS PubMed.
- V. Rajagopalan, U. Olsson and I. Iliopoulos, Langmuir, 1996, 12, 4378–4384 CrossRef CAS.
- J. Galindo-Alvarez, K. A. Le and V. Sadtler, Colloids Surf., A, 2011, 389, 237 CrossRef CAS PubMed.
- R. Liu, W. Pu, H. Jia, X. Shang, Y. Pan and Z. Yan, Int. J. Polym. Sci., 2014, 875637 Search PubMed.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36, 1558–1628 CrossRef CAS PubMed.
- W. F. Pu, R. Liu, K. Y. Wang, K. X. Li, Z. P. Yan, B. Li and L. Zhao, Ind. Eng. Chem. Res., 2015, 54, 798–807 CrossRef CAS.
- G. L. Smith and C. L. McCormick, Langmuir, 2001, 17, 1719–1725 CrossRef CAS.
- J. F. Argillier, A. Audibert, J. Lecourtier, M. Moan and L. Rousseau, Colloids Surf., A, 1996, 113, 247–257 CrossRef CAS.
- S. Talwar, L. Scanu, S. R. Raghavan and S. A. Khan, Langmuir, 2008, 24, 7797–7802 CrossRef CAS PubMed.
- K. Shinoda, A. Carlsson and B. Lindman, Adv. Colloid Interface Sci., 1996, 64, 253–271 CrossRef CAS.
- J. S. Nambam and J. Philip, J. Colloid Interface Sci., 2012, 366, 88–95 CrossRef CAS PubMed.
- H. ShamsiJazeyi, R. Verduzco and G. J. Hirasaki, Colloids Surf., A, 2014, 453, 162–167 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental part, typical FTIR spectrum and 1HNMR spectrum. See DOI: 10.1039/c5ra13865e |
| This journal is © The Royal Society of Chemistry 2015 |