In vivo biodistribution and toxicity of Gd2O3:Eu3+ nanotubes in mice after intraperitoneal injection
Abstract
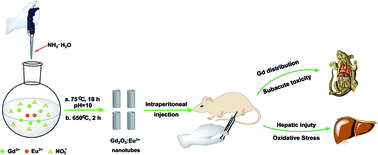
To better understand the potential impact of Eu3+ doped gadolinium oxide nanotubes (Gd2O3:Eu3+ nanotubes) on human health, we investigated their biodistribution, subacute toxicity, and hepatic injury in mice under different dosages (4.0, 40.0, and 400.0 mg kg−1). The results showed that the gadolinium element was mainly accumulated in the spleen, liver, lung, kidney, and bone. The relative organ weight of spleen in the middle-dose group and high-dose group was significant higher than that of the control group. However, the relative organ weight of liver and kidney had no obvious difference from the control group. Besides, the change of toxicity on the hematological system was not noticeable under the tested doses. The high-dose Gd2O3:Eu3+ nanotubes increased the alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels, but no significant difference was observed in the low-dose group and middle-dose group compared with the control group. These changes demonstrated that high-dose Gd2O3:Eu3+ nanotubes induced liver injury. Based on the changes of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), glutathione S-transferase (GST), reactive oxygen species (ROS), malondialdehyde (MDA), and protein carbonylation levels, it can be deduced that high-dose Gd2O3:Eu3+ nanotubes could induce liver injury by oxidative stress. Furthermore, the levels of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-8 (IL-8), increased upon high-dose Gd2O3:Eu3+ nanotube treatment. The results may benefit the applications of Gd2O3:Eu3+ nanotubes.

 Please wait while we load your content...
Please wait while we load your content...