Fabrication of Ag/γ-Fe2O3@TiO2 hollow magnetic core–shell nanospheres as highly efficient catalysts for the synthesis of β-enaminones†
Abstract
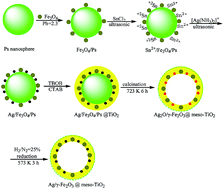
Herein, we describe a method to prepare hollow magnetic mesoporous sphere catalysts (Ag/γ-Fe2O3@meso-TiO2). The core–shell strategy efficiently prevents the aggregation of Ag NPs in the high temperature calcination process and the leaching of Ag NPs for the catalytic reaction in the liquid phase. The catalyst is characterized by TEM, XRD and ICP-AES. Moreover, the catalyst exhibited improved activity for the synthesis of β-enaminones, and it could be easily recovered by an external magnet from the reaction mixture and recycled five times without any significant loss in activity.

 Please wait while we load your content...
Please wait while we load your content...