Single-step synthesis of various distinct hierarchical Ag structures†
Anirban Dandapat‡
a,
Abdul Rahim Ferhan‡a,
Lichan Chena and
Dong-Hwan Kim*ab
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 637457, Singapore
bSchool of Chemical Engineering, Sungkyunkwan University, 16419, Republic of Korea. E-mail: dhkim1@skku.edu
First published on 29th September 2015
Abstract
We introduce a convenient single-step approach for controlled synthesis of unique two- and three-dimensional hierarchical assemblies of silver nanoparticles by utilizing binary and ternary mixtures of capping agents. The binary mixtures comprise of trisodium citrate mixed with either cysteamine or mercaptopropionic acid while the ternary mixtures feature the addition of glucose. We observed the gradual evolution of silver nanoparticle assemblies from a closed-packed arrangement to a flower-like superstructure by simply adjusting the amount of trisodium citrate relative to a fixed amount of cysteamine. The addition of glucose to this binary mixture drastically changes the growth mechanism and instead resulted in nanodisc stacks; this is the first observation of such hierarchical silver nanodisc stacks. On the other hand, the addition of glucose to the binary mixture of mercaptopropionic acid and trisodium citrate produced belt-shaped ensembles with adjustable length. Interestingly, with increasing length, a secondary assembly of silver nanoparticles into petal-shaped structures occurred on the belt surface. While we show that all the structures we prepared can amplify surface enhanced Raman scattering signals, primarily due to their rough morphology, we believe our method can also be extended to other applications, since it provides a simple means to obtain both tuneable and drastically different predefined silver nanoensembles.
Introduction
There is a growing interest in developing metal nanoparticle (NP) ensembles to exploit their collective properties for various prospective applications.1–4 Novel electronic, magnetic and optical properties can arise from the respective interactions between excitons, magnetic moments and surface plasmons of closely-packed assembled nanoparticles. With regard to surface plasmon interactions, gold and silver NP ensembles are particularly sought after in applications involving localized surface plasmon resonance and surface enhanced Raman scattering (SERS). SERS, which relies on electromagnetic field enhancement at nanometer-gap junctions arising from plasmonic coupling of adjacent nanoparticles, represents a highly powerful technique for chemical and biomedical sensing,5–10 allowing detection down to single molecular level.11,12In such applications, it is undoubtedly beneficial for nanoparticles to be arranged in higher-ordered or hierarchical structures, in order to maximize the number of nanometer-gap junctions, as well as to obtain new SERS-enhancing morphologies such as pointed edges and undulating surfaces. This has spurred research on the construction of complex NP assemblies to find new predictable methods for size, shape and morphology control.13,14 Among the approaches developed recently to assemble nanoparticles into various clusters and arrays,6,15–18 self-assembly represents the most promising method for nanoparticle organization into two- and three-dimensional (2D and 3D) hierarchical ensembles. Fine control over the positioning of NP building blocks can be achieved using specific surface capping agents such as alkanethiols. Alkanethiol linking has been recognized as one of the effective strategies for controlled self-assembly,19,20 along with polymer-based molecular recognition,3,21 mediator-template assembly,22,23 as well as hydrogen bonding.24,25 Nonetheless, there is still no established protocol for the controllable construction of 2D and 3D NP assemblies in solution.
In this contribution, we describe the fabrication of various 2D and 3D ensembles of Ag NPs in solution by using binary and ternary mixtures of capping agents, each comprising of a thiolated ligand. Thiolated ligands can form Ag–thiol complexes with free Ag+ ion, decreasing its reduction potential. When this occurs, the reduction process becomes more controllable than the self-assembly process. Based on this, we propose a solution-based method for the controlled assemblies of individual Ag NPs to form 2D and 3D ensembles with tunable shape and morphology. For 2D assembly, as shown in Scheme 1, the individual NPs (i.e. building blocks) can be initially arranged as discrete discs which can be further grown either into thicker disc stacks (isotropic growth) or elongated belts (selective growth along X–Y axis). For the 3D assembly, individual NPs can be grown into either closed-pack (isotropic growth) or opened flower-like (anisotropic growth) structures. We then utilized our structures for SERS measurements by using methylene blue as the probe molecule. All the structures demonstrated SERS enhancing capabilities, with the elongated belts producing the largest enhancement factor.
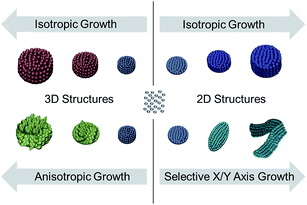 | ||
| Scheme 1 A simplified illustration depicting shape control that lead to the formation of various distinct hierarchical Ag structures featured in this work. | ||
Experimental section
Synthesis of 3D Ag nanostructures
An ethanolic solution of cysteamine was first mixed with an aqueous solution of AgNO3 (1 mL; 10 mM) for 5 min. After that, different amounts of trisodium citrate (SC, 50, 150, 300, 500, 800 and 1500 μL; 30 mM) were added and the solution was stirred for another 5 min. Finally, freshly prepared ascorbic acid (AA) (1 mL; 10 mM) was added under continuous stirring for 1 min at room temperature before finally keeping the solution undisturbed to complete the reaction. The resulting particles were centrifuged and redispersed repeatedly in deionized water and ethanol alternatively to remove excess capping agents. Prior to any characterization, the samples were dried in vacuum to prevent oxidation on the Ag surface.Synthesis of 2D Ag nanostructures
An ethanolic solution of cysteamine/4-mercaptopropionic acid was mixed with an aqueous solution of AgNO3 (1 mL; 10 mM) for 5 min. After that, different amounts of glucose (1.0, 1.5 and 2.0 mL; 30 mM) and SC (0.1 mL; 30 mM) were added and the solution was stirred for another 5 min. Finally, freshly prepared ascorbic acid (AA) (1.0 mL; 10 mM) was added under continuous stirring for 1 min at room temperature before finally keeping the solution undisturbed to complete the reaction. In a later study, cysteamine was replaced by mercaptopropionic acid (MPA, 0.1 and 1.0 mL; 1 mM). For this study different amounts of glucose (0.1, 1.0 and 1.5 mL; 30 mM) and SC (50, 100 and 200 μL; 30 mM) were used. The resulting particles were centrifuged and redispersed repeatedly in deionized water and ethanol alternatively to remove excess capping agents. Prior to any characterization, the samples were dried in vacuum to prevent oxidation on the Ag surface.Characterization
Field-emission scanning electron microscopy (FE-SEM) was performed on the JEOL instrument (JSM-6700F) at an acceleration voltage of 5 kV and a working distance between 7 and 8 mm. Powder X-ray diffraction (XRD) pattern was recorded on a Philips PW 1140 X-ray diffractometer using Cu-Kα radiation (λ = 1.5406 Å). Raman spectra were collected using a Renishaw inVia confocal Raman spectrometer mounted on a Leica microscope with 50× objective lens (NA = 0.80) in the range of 100–2000 cm−1 with one accumulation and 10 s exposure time. A 633 nm wavelength HeNe laser (85 μW at the sample surface) was used to excite the sample.Results and discussion
A series of distinct highly organized 2D and 3D Ag nanostructures were obtained from the simultaneous reduction of silver ions and guided growth of silver nanoparticles. This was achieved via single-step wet chemical synthesis using mixtures of capping agents comprising of trisodium citrate mixed with a thiolated ligand of either cysteamine or mercaptopropionic, both in the presence and absence of glucose. Synthesis in a binary mixture of cysteamine and SC produced 3D hierarchical Ag nanostructures with morphology tunable by varying the amounts of SC, while keeping all other volumes and concentrations constant. While the overall spherical shape of the hierarchical structure is somewhat retained throughout the variation, a radical transformation occurred on the surface resulting in prune-like (Fig. 1a) to flower-like (Fig. 1b) morphologies. Upon further investigation, we found that slight variations in the amounts of SC were sufficient to cause a drastic structural evolution. We began with the addition of 50 μL 30 mM SC, which resulted in a spherical ensemble of closely packed Ag NPs (Fig. 1c). Upon increasing the amount of SC to 150 μL, undulations and ridges appear on the surface resulting in an overall prune-like morphology (Fig. 1d). The ridges deepen with higher volumes of SC (Fig. 1e and f), eventually resulting in a flower-like morphology with sharp, perfectly flat petals (Fig. 1g). Interestingly, when 1 mL SC of the same concentration was used, no 3D hierarchical structure with an overall spherical shape could be formed. Instead, randomly arranged sheets of 2D assembled Ag NPs, which resembled broken petals, were obtained (Fig. 1h). A similitude to this structural evolution is the blooming of a flower, which begins with a bud and ends with opened and finally withered petals. Briefly, this ‘blooming’ process can be accounted by the increasing repulsion between Ag NP building blocks, as a result of an increasing amount of SC (Scheme 2). XRD pattern (ESI; Fig. S1†) of the nanoflowers (Fig. 1b) show sharp peaks at 2θ ∼ 38.1, 44.3, 64.5, 77.4 and 81.5°, which can be well indexed to the (111), (200), (220), (311) and (222) planes, respectively. In other words, the Ag crystals possess a face centred cubic (fcc) structure, confirming the well-crystalline nature of the developed hierarchical nanostructures.26,27 In order to confirm the necessity of having both cysteamine and SC to form these structures, we conducted experiments in the absence of one another. In the absence of cysteamine, only smooth Ag nanoparticles with irregular shape and size were synthesized (Fig. S2†), whereas in the absence of SC, only small agglomerates (80 to 120 nm in diameter) were formed (Fig. S3†).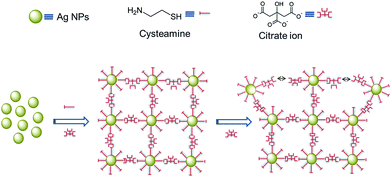 | ||
| Scheme 2 Proposed mechanism describing the morphological transformation of the hierarchical structures from prune-like to flower-like, by using a binary mixture of cysteamine and SC. | ||
As with previous works,28–30 the exact mechanism behind the formation of hierarchical nanostructures is difficult to unravel. In our case, we believe that small Ag NPs (∼5–10 nm) are initially formed via nucleation as soon as Ag+ ions are reduced to atomic Ag. Almost simultaneously, these Ag NPs become building blocks for the formation of higher ordered structures, as they self-assemble into position, depending on their surface chemistry. In a binary mixture of cysteamine and SC, the Ag NP surface will be capped with cysteamine since the –SH group can bind strongly to Ag. The –NH2 group at the exposed end will then attract the carboxylate groups of SC, based on electrostatic interactions. Since SC has three units of carboxylate groups, it can also bind to another –NH2 group from cysteamine attached to a neighbouring Ag NP. This brings together Ag NPs within the same locality, eventually forming a closed packed spherical ensemble (Scheme 2). However, when the amount of SC is increased, instead of acting as a linker between two –NH2 ends extending from neighbouring Ag NPs, they surround the NPs excessively, resulting in NPs capped with cysteamine-SC. This will cause repulsion between neighbouring NPs, leading to the formation of ridges and gaps. Higher amounts of SC eventually lead to the formation of a more open structure with flower-like morphology. Finally, when all the Ag NPs are completely surrounded by SC, a totally repulsive environment restricts the self-organization of the petals into a 3D ensemble, resulting in randomly arranged individual sheets (Fig. 1h).
We then proceeded to extend the principle of capping agent-guided Ag NP assembly to involve a third component, using a ternary mixture of cysteamine, SC and glucose. Interestingly, variation in the amount of glucose can likewise transform the shape of the hierarchical structures drastically; low amount of glucose resulted in disc-like 2D structures while high amount of glucose resulted in belt-like structures. This can be attributed to predominant growth in a single plane (Scheme 1). We observed the transformation in stages starting with 1 mL of 30 mM glucose in a fixed amount of cysteamine (1 mL, 1 mM) and SC (100 μL, 30 mM), which gave sub-micron elongated nanostructures. When we increased the amount of glucose to 1.5 mL, the length of the particles increased to 1–2 μm (Fig. 2c and d). Further increase up to 2 mL glucose led to the formation of belt-shaped hierarchical structures more than 3 microns in length (Fig. 2e and f). In addition, the increase in length was accompanied by an increase in surface roughness manifested as discrete protrusions growing vertically out of the surface plane. Upon closer inspection, these protrusions were made up of small Ag NPs (5–20 nm in diameter). We then varied the amount of SC at fixed amounts of glucose and cysteamine. We observed that lowering the amount of SC would result in more dispersed protrusions made up of plenty but smaller Ag NP aggregates (ESI; Fig. S4†). On the other hand, increasing the amount SC would eliminate the protrusions almost entirely, resulting in belt-like structures with smooth surfaces (ESI; Fig. S5†).
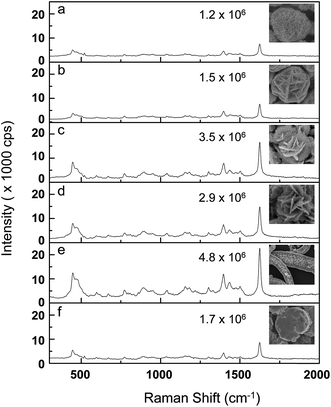
We proceeded to replace the amino terminated thiolated ligand, cysteamine with mercaptopropionic acid (MPA), a carboxylic acid terminated thiolated ligand, in order to observe the significance of the exposed functional group. The generated structures were similar to the ones obtained earlier (i.e. spherical structures with flower-like morphology). However, unlike the case of cysteamine and SC (Fig. 1), the binary mixture of MPA and SC consistently produced these structures, albeit with decreasing size, when the added amount of SC was widely varied from 50 to 200 μL (ESI; Fig. S6†). We believe the absence of attractive forces with the likes of that between cysteamine and SC (Scheme 2) had restricted the formation of a closed pack structure. Instead, repulsion between the negatively charged carboxylate groups of both SC and MPA promotes the development of a flower-like morphology even at very low SC concentrations. The addition of glucose produced disc nanostructures resembling flattened flowers with radially extending petals (Fig. 3). When the amount of added glucose (concentration fixed at 30 mM) was varied from 0.1–1.0 mL, these branched discs gradually became thicker (Fig. 3a–d). When 1.5 mL of glucose was added, a hierarchical assembly of discs (5–6 μm in diameter) was obtained. They are arranged as stacks, each with overall an overall thickness of around 2–3 microns. Although a few variations of Ag nanoplate have been reported,31–33 this is the first observation of hierarchical noble metal nanoplate stacks, obtained from a single-step wet chemical approach. Since all of the hierarchical structures obtained essentially arise from the organized assembly of small Ag NPs, they are expected to show high SERS activities. The nanogaps between adjacent small Ag NPs that make up the higher order structure serve well as SERS hotspots. Since there are plenty of such gaps on the exposed surface, the probability of localizing analyte molecules within a hotspot vicinity is very high. It is also worthy to note that in our system, surfactants were added solely to control the morphological evolution of our structures, enabling them to attain their final unique shapes; after synthesis, the surfactants were thoroughly washed away so that any molecules introduced thereafter can directly adsorb on the Ag surface. In our case, methylene blue (MB), a commonly used SERS analyte, was used as probe molecule to study the SERS activities of the developed nanostructures. The Raman spectra of MB is dominated by ν(C–C) ring stretching (1624 cm−1), ν(C–H) in-plane ring deformation (1397 cm−1), ν(C–N) stretching (1181 cm−1), β(C–H) in-plane bending (771 cm−1), and δ(C–N–C) skeletal deformations (495 and 445 cm−1).34–36 As shown in Fig. 4, well-resolved Raman bands of MB were obtained from all our nanostructures, even at a concentration as low as 10−8 M. However, peak intensities are different for different nanostructures due to variation in surface morphologies and roughness. Maximum intensity was obtained from the nanobelt-like structures. The SERS enhancement capability of all the different nanostructures we obtained was compared through their estimated enhancement factors. This is calculated by considering the apparent enhancement factor (AEF).14,37 AEF is computed according to the following equation: AEF = ISERS[CNRS]/INRS[CSERS]; where CNRS is the concentration of MB deposited on bare silicon substrate, CSERS is the concentration of the adsorbed molecules on the surface of Ag nanostructures, and INRS and ISERS are the peak intensities corresponding to the peak at 1624 cm−1 in normal Raman spectra (ESI; Fig. S7†) and SERS spectra measured from baseline, respectively. The spectra were taken from 5 different positions and an average value of the intensity was used to calculate the AEF.37–40 The calculated AEF values are included to the corresponding SERS spectra in Fig. 4. The values ranged from 1.2 × 106 to 4.8 × 106, depending on the surface morphology of the nanostructures. The belt-like structures, which possessed the roughest surface arising from plates that extend vertically from the surface, undoubtedly produced the highest enhancement factor. Besides determining the AEF, we also checked the relative standard deviation (RSD) values to determine the reproducibility of the signals arising from our structures (Fig. S8†). Likewise, the belt-like structures also produced the best RSD value of 3.1%, while the discrete Ag structures gave the poorest RSD value of 9.5%. Nonetheless, the fact that all RSD values fall below 10% signified the good reproducibility of our structures.
In summary, we have demonstrated a facile single-step, wet chemical approach to guide the assembly of Ag NPs into various distinct hierarchical structures which included spherical ensembles with prune-like and flower-like surface morphologies, elongated belt structures with secondary formation of Ag NP plates vertically extending from the surface, as well as individual discs and disc stacks. This was achieved using binary and ternary mixtures of capping agents with various compositions. Thiol-bearing capping agents, which can form silver–thiolate complexes with lower reduction potential than free Ag+ ions, play an important role in determining the size of the resulting hierarchical structures. Functional groups on the opposite end of the thiolated capping agents determine the surface morphology of the structures. The shape and size of the structure can be varied simply by adjusting the capping agent composition in the mixtures. When tested for SERS activity, all the nanostructures produced herein showed excellent SERS enhancement capabilities, especially the unique belt-like structures. This proves the effectiveness of our approach, albeit its simplicity, in producing novel nanostructures which are useful in cutting edge applications. We believe our strategy also holds great potential for the formation of various unobserved noble metal hierarchical structures, which can be used in other applications.
Acknowledgements
We gratefully acknowledge financial support from Defense Acquisition Program Administration and Agency for Defense Development under the contract UD140080GD and the Ministry of Science, ICT & Future Planning of Korea (NRF-2013M3C8A3078512).References
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- M. Giersig and P. Mulvaney, Langmuir, 1993, 9, 3408–3413 CrossRef CAS.
- R. Shenhar, T. B. Norsten and V. M. Rotello, Adv. Mater., 2005, 17, 657–669 CrossRef CAS PubMed.
- Z. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS PubMed.
- H. Im, K. C. Bantz, N. C. Lindquist, C. L. Haynes and S. H. Oh, Nano Lett., 2010, 10, 2231–2236 CrossRef CAS PubMed.
- W. J. Cho, Y. Kim and J. K. Kim, ACS Nano, 2011, 6, 249–255 CrossRef PubMed.
- H. Wang, C. S. Levin and N. J. Halas, J. Am. Chem. Soc., 2005, 127, 14992–14993 CrossRef CAS PubMed.
- N. A. Abu Hatab, J. M. Oran and M. J. Sepaniak, ACS Nano, 2008, 2, 377–385 CrossRef CAS PubMed.
- J. Paczesny, A. Kamińska, W. Adamkiewicz, K. Winkler, K. Sozanski, M. Wadowska, I. Dziecielewski and R. Holyst, Chem. Mater., 2012, 24, 3667–3673 CrossRef CAS.
- H. Tang, G. Meng, Q. Huang, Z. Zhang, Z. Huang and C. Zhu, Adv. Funct. Mater., 2012, 22, 218–224 CrossRef CAS PubMed.
- K. Kneipp, H. Kneipp and J. Kneipp, Acc. Chem. Res., 2006, 39, 443–450 CrossRef CAS PubMed.
- D. K. Lim, K. S. Jeon, H. M. Kim, J. M. Nam and Y. D. Suh, Nat. Mater., 2010, 9, 60–67 CrossRef CAS PubMed.
- A. Dandapat, T. K. Lee, Y. M. Zhang, S. K. Kwak, E. C. Cho and D.-H. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 14793–14800 CAS.
- Y. Huang, A. Dandapat and D.-H. Kim, Nanoscale, 2014, 6, 6478–6481 RSC.
- S. Pal, Z. Deng, B. Ding, H. Yan and Y. Liu, Angew. Chem., 2010, 122, 2760–2764 CrossRef PubMed.
- B. P. S. Chauhan and R. Sardar, Macromolecules, 2004, 37, 5136–5139 CrossRef CAS.
- N. Pazos-Perez, C. S. Wagner, J. M. Romo-Herrera, L. M. Liz-Marzán, F. J. García de Abajo, A. Wittemann, A. Fery and R. A. Alvarez-Puebla, Angew. Chem., Int. Ed., 2012, 51, 12688–12693 CrossRef CAS PubMed.
- A. R. Ferhan and D.-H. Kim, J. Mater. Chem., 2012, 22, 1274–1277 RSC.
- F. Osterloh, H. Hiramatsu, R. Porter and T. Guo, Langmuir, 2004, 20, 5553–5558 CrossRef CAS.
- J. Wei, N. Schaeffer and M. P. Pileni, J. Phys. Chem. B, 2014, 118, 14070–14075 CrossRef CAS PubMed.
- A. K. Boal, F. Ilhan, J. E. Derouchey, T. Thurn-Albrecht, T. P. Russell and V. M. Rotello, Nature, 2000, 404, 746–748 CrossRef CAS PubMed.
- M. M. Maye, I. I. S. Lim, J. Luo, Z. Rab, D. Rabinovich, T. Liu and C. J. Zhong, J. Am. Chem. Soc., 2005, 127, 1519–1529 CrossRef CAS PubMed.
- I. I. S. Lim, M. M. Maye, J. Luo and C. J. Zhong, J. Phys. Chem. B, 2005, 109, 2578–2583 CrossRef CAS PubMed.
- J. B. Carroll, B. L. Frankamp and V. M. Rotello, Chem. Commun., 2002, 1892–1893, 10.1039/b203771h.
- K. Heo, C. Miesch, T. Emrick and R. C. Hayward, Nano Lett., 2013, 13, 5297–5302 CrossRef CAS PubMed.
- T. Y. Kim, W. J. Kim, S. H. Hong, J. E. Kim and K. S. Suh, Angew. Chem., Int. Ed., 2009, 121, 3864–3867 CrossRef PubMed.
- L. Qi, B. I. Lee, S. Chen, W. D. Samuels and G. J. Exarhos, Adv. Mater., 2005, 17, 1777–1781 CrossRef CAS PubMed.
- B. Zhang, P. Xu, X. Xie, H. Wei, Z. Li, N. H. Mack, X. Han, H. Xu and H. L. Wang, J. Mater. Chem., 2011, 21, 2495–2501 RSC.
- S. Zeng, K. Tang, T. Li, Z. Liang, D. Wang, Y. Wang, Y. Qi and W. Zhou, J. Phys. Chem. C, 2008, 112, 4836–4843 CAS.
- S. Ma, J. Liang, J. W. Zhao and B. Xu, CrystEngComm, 2010, 12, 750–754 RSC.
- D. Jana, A. Mandal and G. de, ACS Appl. Mater. Interfaces, 2012, 4, 3330–3334 CAS.
- X. Y. Zhang, A. Hu, T. Zhang, W. Lei, X. J. Xue, Y. Zhou and W. W. Duley, ACS Nano, 2011, 5, 9082–9092 CrossRef CAS PubMed.
- Y. Sun, B. Mayers and Y. Xia, Nano Lett., 2003, 3, 675–679 CrossRef CAS.
- C. D'Andrea, J. Bochterle, A. Toma, C. Huck, F. Neubrech, E. Messina, B. Fazio, O. M. Maragò, E. Di Fabrizio, M. Lamy de La Chapelle, P. G. Gucciardi and A. Pucci, ACS Nano, 2013, 7, 3522–3531 CrossRef PubMed.
- H. Gehan, L. Fillaud, M. M. Chehimi, J. Aubard, A. Hohenau, N. Felidj and C. Mangeney, ACS Nano, 2010, 4, 6491–6500 CrossRef CAS PubMed.
- G. N. Xiao and S. Q. Man, Chem. Phys. Lett., 2007, 447, 305–309 CrossRef CAS PubMed.
- M. Pradhan, J. Chowdhury, S. Sarkar, A. K. Sinha and T. Pal, J. Phys. Chem. C, 2012, 116, 24301–24313 CAS.
- A. Dandapat, S. Pramanik, S. Bysakh and G. de, J. Nanopart. Res., 2013, 15, 1–10 CrossRef.
- Z. Y. Bao, X. Liu, J. Dai, Y. Wu, Y. H. Tsang and D. Y. Lei, Appl. Surf. Sci., 2014, 301, 351–357 CrossRef CAS PubMed.
- Z. Y. Bao, D. Y. Lei, R. Jiang, X. Liu, J. Dai, J. Wang, H. L. W. Chan and Y. H. Tsang, Nanoscale, 2014, 6, 9063–9070 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13780b |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |