Lithium–oxygen batteries with ester-functionalized ionic liquid-based electrolytes†
Abstract
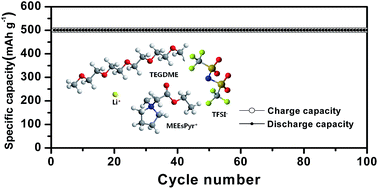
Ester-functionalized ionic liquids with different cations (imidazolium, pyrrolidinium, piperidinium, morpholinium) and a bis(trifluoromethanesulfonyl)imide anion were synthesized and subsequently mixed with tetra(ethylene glycol)dimethylether (TEGDME) at different concentrations. The mixed solutions were ultimately investigated for their applicability as the electrolyte in non-aqueous lithium–oxygen batteries. Among the ionic liquids investigated, the pyrrolidinium-based ionic liquid, ethyl-N-methylpyrrolidinium-N-acetate bis(trifluoromethanesulfonyl)imide (MEEsPyr-TFSI), exhibited a lower viscosity, higher ionic conductivity and wider electrochemical stability window than any other ionic liquids. The addition of an appropriate amount of MEEsPyr-TFSI to the TEGDME-based organic electrolyte had a positive effect on the ionic conductivity, electrochemical stability and oxygen radical stability. A mixed ionic liquid-based solution with an optimum composition was successfully employed as a promising electrolyte for lithium–oxygen batteries with good cycling stability.

 Please wait while we load your content...
Please wait while we load your content...