Synergistic interactions between POSS and fumed silica and their effect on the properties of crosslinked PDMS nanocomposite membranes
Abstract
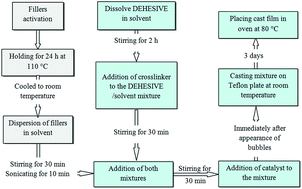
A facile strategy for the synthesis of binary filler nanocomposite membranes containing fumed silica (FS) and octatrimethylsiloxy polyhedral oligomeric silsesquioxane (POSS) nanoparticles was proposed to prepare high performance PDMS–FS–POSS nanocomposite membranes. To fully explore the synergistic effect between the POSS and FS nanoparticles, thermal stability using thermo-gravimetric analysis (TGA) and dispersion quality using scanning electron microscopy (SEM) were investigated, while the crosslinked network was studied using Fourier transformed infrared spectroscopy (FTIR). The results showed that the thermal stability of these novel nanocomposite membranes was much better than that of the neat membrane. Thermodynamically, dipole–dipole interactions between the functional groups are the main parameter leading to better dispersion and thermal stability. Furthermore, it was found that the separation properties of different gases (H2, C3H8, CO2 and CH4) across the nanocomposite membranes were enhanced with increasing FS content. All the improvements observed can be attributed to the synergistic interactions between FS and POSS.

 Please wait while we load your content...
Please wait while we load your content...