Electrochemical behavior of La(III) on liquid Bi electrode in LiCl–KCl melts. Determination of thermodynamic properties of La–Bi and Li–Bi intermetallic compounds
Mei
Li
,
Qunqun
Gu
,
Wei
Han
*,
Xingmei
Zhang
,
Yang
Sun
,
Milin
Zhang
and
Yongde
Yan
Key Laboratory of Superlight Materials and Surface Technology, Ministry of Education, College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China. E-mail: weihan@hrbeu.edu.cn; Fax: +86 451 8253 3026; Tel: +86 451 8256 9890
First published on 24th September 2015
Abstract
Electrochemical behavior of La(III) was studied on liquid Bi electrodes (i.e. Bi pool and Bi coated W electrodes) in LiCl–KCl melts using cyclic voltammetry, square wave voltammetry, chronopotentiometry and open circuit chronopotentiometry. In both the electrodes, the electrochemical reduction of La(III) was observed at more positive potential values than that on an inert W electrode, due to the lowering of the activity of La in liquid Bi phase. Cyclic voltammogram, using a Bi pool electrode, suggests that the reduction of La(III) to Lain liquid Bi was a quasi-reversible and diffusion controlled process. The diffusion coefficient of La in liquid Bi metal was measured by chronopotentiometry at 773 K and was evaluated by using the Sutherland–Einstein equation, respectively. Both the values of the diffusion coefficient were of the same order of magnitude. From the cyclic voltammogram and square wave voltammogram obtained on the Bi coated W electrode, five couples of cathodic/anodic peaks were observed at more positive potential than that for La metal on an inert electrode due to the formation of five La–Bi intermetallic compounds. Thermodynamic properties such as activities and relative partial molar Gibbs energies of M (M = La, Li) in the M–Bi alloys as well as Gibbs energies of formation for M–Bi (M = La, Li) intermetallic compounds, LaBi2, LaBi, La4Bi3, La5Bi3, La2Bi and Li3Bi were calculated from the open circuit potential measurement. La–Bi and La–Li–Bi alloys were produced on liquid Bi pool electrodes by galvanostatic and potentiostatic electrolysis, and characterized by X-ray diffraction (XRD) and scanning electronic microscopy (SEM). The results indicated that La–Bi alloys were comprised of LaBi2, LaBi and La2Bi phases, and LaBi2, LaBi and Li3Bi phases existed in La–Li–Bi alloys.
Introduction
The pyrometallurgical process is one of the most promising options for the reprocessing of advanced nuclear fuels and transmutation blankets which have high burn up potential and are expected to have a high Pu and minor actinide contents.1–4 Electrorefining5–8 and liquid–liquid metal reductive extraction8,9 are the most developed pyrochemical techniques intended for separation of actinides from fission products in molten salt media. Since the use of liquid metal electrodes has many advantages, such as complete physical separation between the electrolyte and the produced metal, constant surface area of the electrode, and easier coalescence of microdrops and metallic fog, the studies on liquid metals as working electrode are being extensively used to nuclear applications for pyrochemical reprocessing of irradiated nuclear fuels.10–24 Gibilaro et al.10 investigated the electrochemical properties of various liquid metallic electrodes (Bi, Sb, Sn, Pb, Ga) in LiF–NaF and LiF–CaF2 melts. They found the reactivity and nobility scale of liquid metallic electrodes are in the order: Sb > Bi > Pb > Sn > Ga > Mo and Ga < Sn < Pb < Sb < Bi < Mo, respectively, by linear sweep voltammetry techniques. Murakami and Koyama11 evaluated the diffusion coefficients of rare-earths (La, Pr, Nd, Gd, Y and Sc) in liquid Cd metal from the results of chronopotentiometry. The electrochemical behavior of Ce(III),12 Pr(III),13 Nd(III),14 Tb(III),15 U(III),16,17 Pu(III)17,18 and Np(III)19 were investigated on a liquid Cd electrode in molten LiCl–KCl eutectic. Castrillejo et al.13,15 evaluated the thermodynamic properties of the formation for the Tb–Cd and Pr–Cd intermetallic compounds by emf measurements. Vandarkuzhali et al.14 studied the apparent standard electrode potentials of Nd(III)/Nd for different temperatures in the range 698–773 K. The formation of intermetallic, Cd11Nd, was studied from open circuit potential measurement on a Cd film electrode. Kato et al.20 investigated the separation behavior of actinides from rare-earths in molten salt electrorefining using saturated liquid cadmium cathode. The separation factors were calculated, and the values of separation factors were 0.011, 0.044, 0.064, and 0.064 for La, Ce, Pr, and Nd, respectively. Castrillejo et al.13,21 explored the electrode reactions of LiCl–KCl–PrCl3 and LiCl–KCl–CeCl3 solutions at the surface of liquid Bi electrode by electrochemical techniques. Emf measurements on various intermetallic compounds in two-phase coexisting states were carried out. The thermodynamic properties of formation for intermetallic compounds of Pr–Bi and Ce–Bi were calculated, respectively. Shirai et al.19,22 studied the electrochemical behaviors of Pu(III)/Pu and Np(III)/Np couples at the interface between the LiCl–KCl and liquid Bi metal. The electrochemical behavior of the Th(IV)/Th system was examined in molten LiF–CaF2 medium23 and LiCl–KCl eutectic24 on a liquid Bi film electrode, and the Bi–Th alloys were obtained. To the best of our knowledge, fundamental studies on the mechanism of electrode reaction and thermochemical data are not much reported on liquid Bi electrode.Lanthanum, as the first element in the lanthanide series, having the closest ionic radius to U(III) among lanthanides, is a typical fission product and difficult to be separated from actinides. Therefore, it has been extensively studied on different cathodes, such as W,25–28 Mo,29,30 Al,28 Cd11,20,31 and Bi film32 electrodes in molten salts. Serp et al.32 studied the electroseparation of plutonium from lanthanum using a Bi film electrode in LiCl–KCl eutectic at 733 K. The reduction potentials of Pu(III) and La(III) ions were measured on a Bi thin film electrode using cyclic voltammetry. They found that the difference between the peak potentials for the formation of PuBi2 and LaBi2 was approximately 100 mV and Pu could be efficiently deposited and separated from lanthanum.
However, there is sparse information about the electrochemical behaviors of La on liquid electrodes. In order to better understand the electrochemical reactions of La(III) on liquid electrodes, further acquisition of basic electrochemical and thermodynamic data on La in LiCl–KCl system is needed. Therefore, in the present paper, we explored the electrochemical behavior of La(III) on a liquid Bi pool electrode and a Bi coated W electrode, the diffusion coefficients of La in liquid Bi metal, as well as the thermodynamic properties of La–Bi and Li–Bi intermetallic compounds were determined by means of electrochemical techniques.
Experimental
Preparation and purification of the melts
The eutectic mixture of LiCl–KCl (45.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.2 mass, analytical grade) was first dried under vacuum for more than 72 h at 473 K to remove excess water and then melted in an alumina crucible placed in a quartz cell located in an electric furnace. Other impurities in the melts were removed by pre-electrolysis at −2.0 V (vs. Ag/AgCl) for 3 h. La(III) ions were introduced into the bath in the form of dehydrated LaCl3 powder (analytical grade). Since Ln ions are very sensitive to O2− ions, in order to avoid the oxidation of La(III), HCl was bubbled to the melts to purify the melts and then argon was bubbled to remove the excess HCl.33 The temperature of the melts was measured with a nickel chromium–nickel aluminum thermocouple sheathed by an alumina tube. All experiments were performed under an argon atmosphere.
54.2 mass, analytical grade) was first dried under vacuum for more than 72 h at 473 K to remove excess water and then melted in an alumina crucible placed in a quartz cell located in an electric furnace. Other impurities in the melts were removed by pre-electrolysis at −2.0 V (vs. Ag/AgCl) for 3 h. La(III) ions were introduced into the bath in the form of dehydrated LaCl3 powder (analytical grade). Since Ln ions are very sensitive to O2− ions, in order to avoid the oxidation of La(III), HCl was bubbled to the melts to purify the melts and then argon was bubbled to remove the excess HCl.33 The temperature of the melts was measured with a nickel chromium–nickel aluminum thermocouple sheathed by an alumina tube. All experiments were performed under an argon atmosphere.
Electrochemical apparatus and electrodes
All electrochemical measurements were performed using an Autolab PGSTAT 302N (Metrohm, Ltd) with Nova 1.8 software where techniques of cyclic voltammetry (CV), square wave voltammetry (SWV), chronopotentiometry (CP) and open circuit chronopotentiometry (OCP) were employed. A silver wire (d = 1 mm) dipped into a solution of AgCl (1 wt%) in the LiCl–KCl melts contained in a Pyrex tube was used as the reference electrode. All potentials were referred to this Ag/AgCl couple. Different working electrodes have been used: (i) Bi pool electrode, (ii) liquid Bi electrode containing La, (iii) tungsten wire (d = 1 mm, 99.99%) in order to compare the results with the liquid electrode. A spectral pure graphite rod (d = 6 mm) was used as the counter electrode.The liquid Bi pool electrode (shown in Fig. 1) was prepared by placing some granules of Bi (99.999%) in a J shaped Pyrex tube, which was immersed in the LiCl–KCl melts. Contact was established by using a W wire (d = 1 mm, 99.99%) immersed in the liquid Bi phase through the Pyrex tube.
The liquid Bi electrode containing La was prepared by potentiostatic electrolysis. The concentration of La in liquid Bi phase (CLa) was kept lower than its solubility. Assuming that the current efficiency of reaction on a liquid Bi pool electrode would be 100%, CLa could be calculated. The CLa values measured by inductively coupled plasma-atomic emission spectrometer (ICP-AES) after experiments, agreed well with the values calculated under the above assumption. Thus the assumption was considered to be appropriate.
Molten salts electrolysis and characterization of deposits
The deposits were prepared by potentiostatic and galvanostatic electrolysis, respectively. After electrolysis, the alloy samples were washed in hexane (99.8%) in an ultrasonic bath to remove salts and stored in a glove box for analysis. These deposits were analyzed by X-ray diffraction (XRD, X'Pert Pro; Philips Co., Ltd) using Cu Kα radiation at 40 kV and 40 mA. The specimen was mounted in thermosetting resins using a metallographic mounting press and then mechanically polished. And then, the microstructure was measured using scanning electronic microscopy (SEM, JSM-6480A; JEOL Co., Ltd).Results and discussion
The electrochemical behavior of La(III) on a liquid Bi pool electrode in LiCl–KCl melts
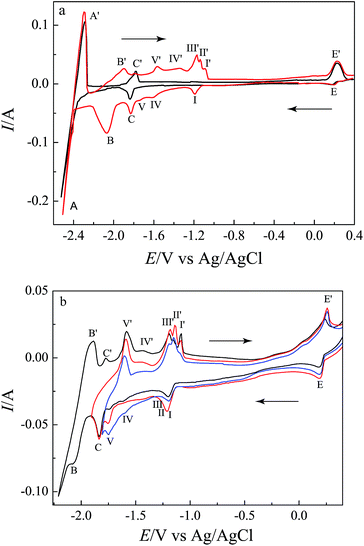
Fig. 2 presents the cyclic voltammograms obtained in LiCl–KCl melts on a W electrode and a liquid Bi pool electrode before and after the addition of (5.2 × 10−5 mol cm−3) LaCl3, respectively. In curve a (black line), one couple of cathodic/anodic signals is observed at approximately −2.40/−2.22 V (vs. Ag/AgCl). In such a negative potential, only metal cation/metal can obtain/lose electron in LiCl–KCl melts. Thus, the cathodic/anodic signals, A/A′, correspond to the reduction of Li(I) and the re-oxidation of Li metal, respectively.
Curve b (red line) shows the cyclic voltammograms of LaCl3 (5.2 × 10−5 mol cm−3) in LiCl–KCl melts on a W electrode at 773 K. The signals B/B′, at −2.04/−1.98 V, correspond to the deposition of La(III) and dissolution of La, respectively, which are consistent with those obtained by Tang and Pesic.30
| La(III) + 3e− → La | (1) |
The typical cyclic voltammograms of LiCl–KCl melts on a liquid Bi electrode is presented in curve c (green line). Compared with curve a (black line), two new pairs of reduction/oxidation peaks are observed. Except the E′ corresponding to the anodic dissolution of liquid Bi electrode, the signals C/C′, at −1.40/−1.31 V, should be attributed to the deposition of Bi–Li alloy and subsequent dissolution of deposits.21
| Li(I) + e− → Liin liquid Bi | (2) |
After the addition of LaCl3 in LiCl–KCl melts, the typical cyclic voltammograms obtained on a liquid Bi electrode is presented in curve d (blue line). The voltammograms consist of a single cathodic wave D, at −1.13 V, associated with an anodic wave D′, at −0.98 V, at potential values less cathodic than those obtained on an inert W electrode, due to a lowering of the activity of the dissolved La in liquid Bi phase as described in eqn (4).
| La(III) + 3e− → Lain liquid Bi | (3) |
 | (4) |
The shape of the voltammograms is that expected for a soluble–soluble exchange. The results also suggest that the solubility of La in the liquid Bi metal has not been achieved in the potential region below −1.2 V. A similar conclusion has been reported by Castrillejo et al.21 in the case of Ce(III) electroreduction in liquid Bi metal.
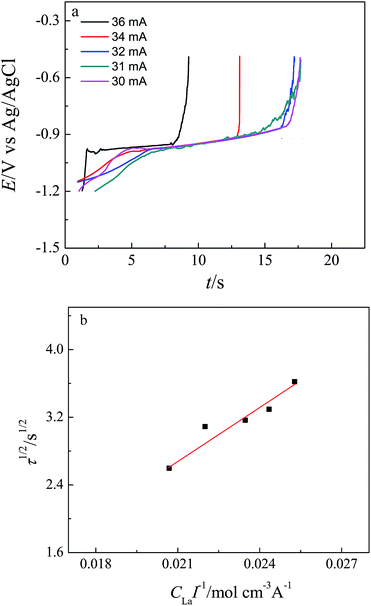
Fig. 3 shows a series of the voltammograms obtained in LiCl–KCl–LaCl3 melts on a Bi pool electrode, without formation of intermetallic compounds, at different scan rates. Furthermore, a further analysis of the recorded voltammograms is based on the measurement of the variation of peak currents and peak potentials with scan rates. Fig. 4a presents the linear relationship between the reduction peak current and the square root of the scan rate. The straight line through the origin proved that the reduction process is limited by mass transport both in the salt and the metallic phase. The reversibility of the system is examined by plotting the influence of the scan rate on the peak potential as presented in the Fig. 4b. At scan rate lower than 0.03 V s−1, the peak potential, EP, is constant and independent of the scan potential rate. Whereas for higher scan rates, the values of the anodic and cathodic peak potentials shift slightly towards positive and negative ones. These results indicate that the reduction of La(III) on a liquid Bi electrode is quasi-reversible above 0.03 V s−1 scan rate.
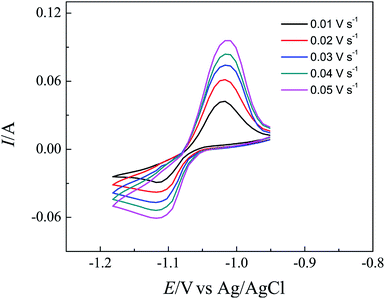 | ||
| Fig. 3 Cyclic voltammograms obtained in LiCl–KCl–LaCl3 (5.2 × 10−5 mol cm−3) melts on a liquid Bi pool electrode (S = 0.2 cm2) at different scan rates at 773 K. | ||
 | (5) |
| Applied current/A | C La × 10−4 mol cm−3 | τ/s |
|---|---|---|
| 0.03 | 7.584 | 13.114 |
| 0.031 | 7.545 | 10.856 |
| 0.032 | 7.511 | 10.03 |
| 0.034 | 7.479 | 9.55 |
| 0.036 | 7.447 | 6.75 |
We also estimated the diffusion coefficient of La in liquid Bi metal, according to Sutherland–Einstein equation:35
| D = kT/4πrη | (6) |
| ηBi = 4.458 × 10−4e775.8/T | (7) |
Combining eqn (6) and (7), the value of diffusion coefficient of La (DLa) in liquid Bi was calculated to be 3.72 × 10−5 cm2 s−1 which is in the same order of magnitude to that obtained by chronopotentiometry.
Electrochemical behavior of La(III) on a Bi coated W electrode in LiCl–KCl–LaCl3 melts
According to the La–Bi binary phase diagram,37 there are five La–Bi intermetallic compounds, LaBi2, LaBi, La4Bi3, La5Bi3 and La2Bi in the La–Bi system. In order to obtain thermodynamic information concerning formation process of La–Bi intermetallic compounds, the electrochemical behavior of La(III) on a W electrode coated with a thin film of Bi has been studied by cyclic voltammetry, square wave voltammetry, and open circuit chronopotentiometry.After the addition of LaCl3 (8.67 × 10−5 mol cm−3) in LiCl–KCl–BiCl3 melts (red line in Fig. 6a), the voltammogram is more complex than that obtained on a liquid Bi pool electrode. In addition to the peaks A/A′, C/C′ and E/E′, peaks B/B′ can be ascribed to the reduction of La(III) to metal and its subsequent re-oxidation, respectively, according to the above results of LiCl–KCl–LaCl3 melts on a W electrode (red line in Fig. 2). Between the peaks B/B′ and E/E′, a series of redox couples are ascribed to the formation/dissolution of different intermetallic compounds. In order to associate the dissolution anodic peaks with their corresponding cathodic formation, the voltammograms were registered at different inversion cathodic potentials, and the results are shown in Fig. 6b. Five new anodic peaks, I′, II′, III′, IV′ and V′ observed correspond to the formation of five La–Bi intermetallic compounds, respectively. Since reduction peak potentials of I, II, and III are very close, the three cathodic peaks overlap each other. We can't distinguish the three cathodic peaks, respectively.
Open circuit chronopotentiometry. – Thermodynamic properties of La–Bi intermetallic compounds
Adopting open circuit chronopotentiometry, as an appropriate electrochemical technique for the study of underlying alloys formation and dissolution, a thin layer specimen was prepared by cathodic deposition on a W electrode for short periods in the melts. The open-circuit potential of the electrode was registered vs. time (as shown in Fig. 8). During this process, potential plateaus are associated with the state where two phases coexist at the electrode surface. Curve a (red curve) in Fig. 8 shows an open circuit chronopotentiogram obtained in the LiCl–KCl–BiCl3 (3.37 × 10−6 mol cm−3) melts on a W electrode while applying a deposition potential of −2.50 V for 60 s. In the beginning, the potential stays at around −2.35 V (plateau A), which is interpreted due to the presence of deposited Li metal on the electrode and related to the Li(I)/Li(0) redox couple. After that, plateau C is correlated with the formation of Li–Bi alloy. According to the below electrolysis results, the Li–Bi intermetallic compound is Li3Bi characterized by XRD. The rest potential at 0.20 V is associated with the Bi(III)/Bi(0) system. Curve b (black curve) presents an open circuit chronopotentiogram obtained in the LiCl–KCl–LaCl3 (8.67 × 10−5 mol cm−3) melts on a W electrode with applying a deposition potential of −2.50 V for 60 s. Except for the plateau A, a new plateau B observed, is ascribed to the La(III)/La(0) redox couple. Curve c (blue curve) illustrates the open circuit chronopotentiogram carried out in the LiCl–KCl–LaCl3 (8.67 × 10−5 mol cm−3)–BiCl3 (3.37 × 10−6 mol cm−3) melts after affording a deposition potential of −2.5 V for 60 s. In curve c, five new plateaus, I, II, II, IV and V, are observed, corresponding to the two phases coexisting of five different La–Bi intermetallic compounds, respectively.The equilibrium potentials measured with reference to the Ag/AgCl couple were converted to the electromotive forces (emf) against M(0) (M = La, Li), making it possible to estimate the standard molar Gibbs energies of formation for M–Bi (M = La, Li) intermetallics.12,13,21,38
Therefore the plateaus (in Fig. 8) could be considered to correspond to the following reactions:
| Plateau A: Li(I) + e− ↔ Li | (8) |
| Plateau B: La(III) + 3e− ↔ La | (9) |
| Plateau C: Li(I) + e− + 1/3Bi ↔ LiBi1/3 | (10) |
| Plateau E: Bi(III) + 3e− ↔ Bi | (11) |
| Plateau I: La(III) + 3e− + 2Bi ↔ LaBi2 | (12) |
| Plateau II: La(III) + 3e− + LaBi2 ↔ 2LaBi | (13) |
| Plateau III: La(III) + 3e− + 3LaBi ↔ La4Bi3 | (14) |
| Plateau IV: La(III) + 3e− + La4Bi3 ↔ La5Bi3 | (15) |
| Plateau V: La(III) + 3e− + La5Bi3 ↔ 3La2Bi | (16) |
The emf corresponding to the chemical composition of M–Bi (M = La, Li) alloys is related to the activity of M (M = La, Li), by the expression:
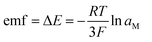 | (17) |
The relative partial molar Gibbs free energies of La in the La–Bi intermetallic compounds, were also calculated from the obtained emf, ΔE vs. (La(III)/La)/V, by the following equations:
ΔḠLa = −3FΔE = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aLa aLa | (18) |
The values obtained at different temperatures are given in Table 2 and Table 3. The aLi in the two-phase coexisting states between Li3Bi and Li was found to be in the order of 10−5 to 10−4 as shown in Table 2. They are larger than the activities of La in La–Bi alloys in Table 3.
| T (K) | E (V) vs. Li(I)/Li | ΔGөf(Li3Bi)/kJ mol−1 | a Li | ΔGөf(T)/kJ mol−1 |
|---|---|---|---|---|
| 723 | 0.584 ± 0.003 | −169.07 ± 0.87 | 8.48 × 10−5 | −225.33 + 0.077T |
| 748 | 0.578 ± 0.005 | −167.33 ± 1.45 | 1.27 × 10−4 | |
| 773 | 0.572 ± 0.004 | −165.59 ± 1.16 | 1.86 × 10−4 | |
| 798 | 0.565 ± 0.003 | −163.57 ± 0.87 | 2.70 × 10−4 | −255.40 + 0.059T (ref. 39) |
| 823 | 0.557 ± 0.004 | −161.25 ± 1.16 | 3.88 × 10−4 |
| T/K | E eq/V vs. Ag/AgCl | ΔE/V vs. La(III)/La | ΔḠLa/kJ (mol La)−1 | a La |
|---|---|---|---|---|
| Plateau (La) | ||||
| 723 | −1.968 ± 0.003 | |||
| 748 | −1.961 ± 0.003 | |||
| 773 | −1.950 ± 0.002 | |||
| 798 | −1.939 ± 0.002 | |||
| 823 | −1.921 ± 0.001 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Plateau I | ||||
| 723 | −1.122 ± 0.003 | 0.846 ± 0.006 | −244.92 ± 1.74 | 2.03 × 10−18 |
| 748 | −1.118 ± 0.002 | 0.843 ± 0.005 | −244.05 ± 1.45 | 9.08 × 10−18 |
| 773 | −1.109 ± 0.002 | 0.841 ± 0.004 | −243.47 ± 1.16 | 3.54 × 10−17 |
| 798 | −1.102 ± 0.003 | 0.837 ± 0.005 | −242.31 ± 1.45 | 1.38 × 10−16 |
| 823 | −1.099 ± 0.001 | 0.822 ± 0.002 | −237.97 ± 0.58 | 7.86 × 10−16 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Plateau II | ||||
| 723 | −1.188 ± 0.005 | 0.78 ± 0.008 | −225.81 ± 2.32 | 4.83 × 10−17 |
| 748 | −1.182 ± 0.004 | 0.779 ± 0.007 | −225.52 ± 2.03 | 1.79 × 10−16 |
| 773 | −1.176 ± 0.006 | 0.774 ± 0.008 | −223.49 ± 2.32 | 7.86 × 10−16 |
| 798 | −1.171 ± 0.003 | 0.768 ± 0.005 | −221.47 ± 1.45 | 3.17 × 10−15 |
| 823 | −1.164 ± 0.004 | 0.757 ± 0.005 | −219.15 ± 1.45 | 1.23 × 10−14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Plateau III | ||||
| 723 | −1.240 ± 0.001 | 0.728 ± 0.004 | −210.76 ± 1.16 | 5.94 × 10−16 |
| 748 | −1.234 ± 0.003 | 0.727 ± 0.006 | −210.47 ± 1.74 | 2.01 × 10−15 |
| 773 | −1.231 ± 0.002 | 0.719 ± 0.004 | −208.15 ± 1.16 | 8.57 × 10−15 |
| 798 | −1.226 ± 0.004 | 0.713 ± 0.006 | −206.41 ± 1.74 | 3.08 × 10−14 |
| 823 | −1.216 ± 0.006 | 0.705 ± 0.007 | −204.10 ± 2.03 | 1.11 × 10−13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Plateau IV | ||||
| 723 | −1.284 ± 0.006 | 0.684 ± 0.009 | −198.02 ± 2.61 | 4.95 × 10−15 |
| 748 | −1.278 ± 0.004 | 0.683 ± 0.007 | −197.73 ± 2.03 | 1.56 × 10−14 |
| 773 | −1.269 ± 0.005 | 0.681 ± 0.007 | −197.15 ± 2.03 | 5.24 × 10−14 |
| 798 | −1.261 ± 0.006 | 0.678 ± 0.008 | −196.28 ± 2.32 | 1.10 × 10−13 |
| 823 | −1.247 ± 0.007 | 0.674 ± 0.008 | −195.12 ± 2.32 | 4.11 × 10−13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Plateau V | ||||
| 723 | −1.656 ± 0.001 | 0.312 ± 0.004 | −90.32 ± 1.16 | 2.97 × 10−7 |
| 748 | −1.650 ± 0.003 | 0.311 ± 0.006 | −90.04 ± 1.74 | 5.15 × 10−7 |
| 773 | −1.644 ± 0.002 | 0.306 ± 0.004 | −88.59 ± 1.16 | 1.04 × 10−6 |
| 798 | −1.642 ± 0.002 | 0.297 ± 0.004 | −85.98 ± 1.16 | 2.35 × 10−6 |
| 823 | −1.639 ± 0.002 | 0.282 ± 0.003 | −81.68 ± 0.87 | 6.33 × 10−6 |
And the standard Gibbs energy of formation of Li3Bi, ΔG0f(Li3Bi) can be calculated from the emf by the relation:
| ΔGөf(Li3Bi) = −3FΔEll | (19) |
The temperature dependence of the emf is measured, and the variation of the Gibbs energy of formation of Li3Bi with the temperature (ΔGӨf(T)) is also presented in Table 2. Weppner and Huggins39 determined the Gibbs energy of formation of Li–Bi intermetallic compounds in the temperature range between 628 K and 873 K by emf method. Compared with their data, our experimental data are different, with a relative deviation of 11%. The possible reasons are attributed to the different experimental condition.
The standard molar Gibbs energies of formation ΔGӨf for La–Bi intermetallic compound are calculated by the equation listed in Table 4. Morisson and Petot40 determined the thermodynamic properties of LaBi2 in CaF2–LaF3 melts in the temperature range of 823–1023 K using emf measurements. The value of Gibbs energy of formation for LaBi2 at 823 K is very close to our experiment result. The linear dependence of the energy of formation, of the different intermetallic compounds, with the temperatures, was also presented in Table 4.
| Intermetallic compound | Equation | T/K | ΔGӨf/kJ mol−1 | ΔGӨf(T)/kJ mol−1 |
|---|---|---|---|---|
| LaBi2 | ΔGӨf(LaBi2) = −3FΔE1 | 723 | −244.92 ± 1.74 | −269.22 + 0.033T |
| 748 | −244.05 ± 1.45 | |||
| 773 | −243.47 ± 1.16 | |||
| 798 | −242.31 ± 1.45 | |||
| 823 | −237.97 ± 0.58, −236.29 (ref. 40) | |||
| LaBi |

|
723 | −235.36 ± 2.03 | −269.55 + 0.047T |
| 748 | −234.79 ± 1.74 | |||
| 773 | −233.48 ± 1.74 | |||
| 798 | −231.89 ± 1.45 | |||
| 823 | −228.56 ± 1.01 | |||
| La4Bi3 | ΔGӨf(La4Bi3) = [3ΔGӨf(LaBi) − 3FΔE3] | 723 | −916.85 ± 7.24 | −1065.20 + 0.20T |
| 748 | −915.11 ± 6.95 | |||
| 773 | −908.60 ± 6.37 | |||
| 798 | −902.08 ± 6.08 | |||
| 823 | −889.78 ± 5.07 | |||
| La5Bi3 | ΔGӨf(La5Bi3) = ΔGӨf(La4Bi3) − 3FΔE4 | 723 | −1114.87 ± 9.84 | −1280.12 + 0.23T |
| 748 | −1112.84 ± 8.97 | |||
| 773 | −1105.75 ± 8.40 | |||
| 798 | −1098.37 ± 8.40 | |||
| 823 | −1084.90 ± 7.38 | |||
| La2Bi |

|
723 | −401.73 ± 3.67 | −470.96 + 0.094T |
| 748 | −400.96 ± 3.57 | |||
| 773 | −398.11 ± 3.18 | |||
| 798 | −394.78 ± 3.19 | |||
| 823 | −388.86 ± 2.75 |
Galvanostatic and potentiostatic electrolysis and characterization of the deposits
To confirm the underpotential deposition of La on liquid Bi, galvanostatic and potentiostatic electrolysis were carried out on a Bi coated W electrode in the LiCl–KCl–LaCl3–BiCl3 melts. The La–Bi alloys prepared were always residue-shaped in molten salts whether at low or high temperature, we could not collect the La–Bi alloys. Therefore, galvanostatic and potentiostatic electrolysis were executed on a liquid Bi pool electrode in the LiCl–KCl–LaCl3 melts. Fig. 9 shows SEM image and XRD pattern of alloy sample obtained by galvanostatic electrolysis (−0.1 A) for 8 h in LiCl–KCl–LaCl3 (3.7 × 10−4 mol cm−3) melts on a liquid Bi pool electrode. The XRD pattern shows that the sample is comprised of LaBi2, LaBi, Li3Bi and Bi phases. Fig. 10 shows XRD pattern of alloy samples obtained by potentiostatic electrolysis at −1.4 V in LiCl–KCl–LaCl3 (3.7 × 10−4 mol cm−3) melts on a liquid Bi pool electrode for 4 h and 6 h, respectively. The XRD patterns show the formation of different La–Bi intermetallic compounds. La-rich compound, La2Bi, can be obtained by potentiostatic electrolysis with increasing the electrolysis time, but intermetallic compounds, La4Bi3 and La5Bi3, do not produced under our experimental conditions.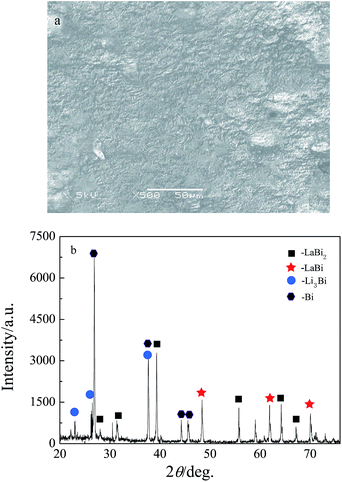 | ||
| Fig. 9 SEM image (a) and XRD pattern (b) of the sample obtained by galvanostatic electrolysis for 8 h at −0.1 A on a liquid Bi pool electrode (S = 1.53 cm2) at 773 K. | ||
Conclusion
The electrochemical behavior of La(III) was investigated in LiCl–KCl–LaCl3 melts using a series of electrochemical techniques on liquid Bi electrodes (i.e. Bi pool and Bi coated W electrodes) at 773 K. The redox potentials of the La(III)/La couple on a liquid Bi electrode were observed at more positive potential values than that on an inert W electrode. This potential shift was due to a lowering of activity of La in liquid Bi phase caused by the formation of La–Bi intermetallic compounds. The diffusion coefficient of La in liquid Bi phase was measured by chronopotentiometry and was found to be 1.81 × 10−5 cm2 s−1.Electromotive force, emf, measurements for different intermetallic compounds in two-phase coexisting states were carried out in the temperature range of 723–823 K. The activities of M (M = La, Li) in the M–Bi intermetallic compounds as well as the Gibbs energy of formation for M–Bi (M = La, Li) intermetallic compounds, LaBi2, LaBi, La4Bi3, La5Bi3, La2Bi, and Li3Bi were obtained. The linear dependence of the standard molar Gibbs energies of formation of M–Bi (M = La, Li) intermetallic compounds with temperature was presented.
La–Bi and La–Li–Bi alloys were directly fabricated by potentiostatic and galvanostatic electrolysis on a liquid Bi pool electrode in LiCl–KCl–LaCl3 melts at 773 K. LaBi2, LaBi and La2Bi phases, characterized by XRD, in bulk La–Bi alloys which were obtained by potentiostatic electrolysis. During the process of galvanostatic electrolysis, La–Li–Bi alloys obtained were comprised of LaBi2, LaBi and Li3Bi phases.
Acknowledgements
The work was financially supported by Key Laboratory of Superlight Materials and Surface Technology, Ministry of Education, the National Natural Science foundation of China (21271054, 11575047 and 21173060), the Major Research plan of the National Natural Science Foundation of China (91326113 and 91226201), the Fundamental Research funds for the Central Universities (HEUCF201503007) and the International Exchange Program of Harbin Engineering University for Innovation-oriented Talents Cultivation.References
- M. Gibilaro, L. Massot, R. Chamelot and P. Taxil, Electrochim. Acta, 2009, 54, 5300–5306 CrossRef CAS PubMed
.
- K. Kinoshita, K. Tadafumi, I. Tadashi, M. Ougier and J. P. Glatz, J. Phys. Chem. Solids, 2005, 66, 619–624 CrossRef CAS PubMed
.
- P. Soucek, L. Cassayre, R. Malmbeck, E. Mendes, R. Jardin and J. P. Glatz, Radiochim. Acta, 2008, 96, 315–322 CrossRef CAS
.
- O. Shirai, T. Iwai, K. Shiozawa, Y. Suzuki, Y. Sakamura and T. Inoue, J. Nucl. Mater., 2000, 277, 226–230 CrossRef CAS
.
- H. F. McFarlane and M. J. Lineberry, Prog. Nucl. Energy, 1997, 31, 111–129 CrossRef
.
- M. Iizuka, K. Uozumi, T. Inoue, T. Iwai, O. Shirai and Y. Arai, J. Nucl. Mater., 2001, 299, 32–42 CrossRef CAS
.
- K. Uozumi, M. Iizuka, T. Kato, T. Inoue, O. Shirai, T. Iwai and Y. Arai, J. Nucl. Mater., 2004, 325, 34–43 CrossRef CAS PubMed
.
- O. Conocar, N. Douyere, J. P. Glatz, J. Lacquement, R. Malmbeck and J. Serp, Nucl. Sci. Eng., 2006, 153, 253–261 CAS
.
- K. Kinoshita, T. Inoue, S. P. Fusselman, D. L. Grimmett, J. J. Roy, R. L. Gay, C. L. Krueger, C. R. Nabelek and T. S. Storvick, J. Nucl. Sci. Technol., 1999, 36, 189–197 CrossRef CAS PubMed
.
- M. Gibilaro, S. Bolmont, L. Massot, L. Latapie and P. Chamelot, J. Electroanal. Chem., 2014, 726, 84–90 CrossRef CAS PubMed
.
- T. Murakami and T. Koyama, J. Electrochem. Soc., 2011, 158, F147–F153 CrossRef CAS PubMed
.
- S. H. Kim, S. Paek, T. J. Kim, D. Y. Park and D. H. Ahn, Electrochim. Acta, 2012, 85, 332–335 CrossRef CAS PubMed
.
- Y. Castrillejo, M. R. Bermejo, P. Díaz Arocas, A. M. Martínez and E. Barrado, J. Electroanal. Chem., 2005, 579, 343–358 CrossRef CAS PubMed
.
- S. Vandarkuzhali, M. Chandra, S. Ghosh, N. Samanta, S. Nedumaran, B. P. Reddy and K. Nagarajan, Electrochim. Acta, 2014, 145, 86–98 CrossRef CAS PubMed
.
- Y. Castrillejo, P. Hernández, R. Fernández and E. Barrado, Electrochim. Acta, 2014, 147, 743–751 CrossRef CAS PubMed
.
- G. Y. Kim, D. Yoon, S. Paek, S. H. Kim, T. J. Kim and D. H. Ahn, J. Electroanal. Chem., 2012, 682, 128–135 CrossRef CAS PubMed
.
- K. Uozomi, M. Iizuka, T. Kato, T. Inoue, O. Shirai, T. Iwai and Y. Arai, J. Nucl. Mater., 2004, 325, 34–43 CrossRef PubMed
.
- O. Shirai, M. Iizuka, T. Iwai, Y. Suzuki and Y. Arai, J. Electroanal. Chem., 2000, 490, 31–36 CrossRef CAS
.
- O. Shirai, K. Uozumi, T. Iwai and Y. Arai, J. Appl. Electrochem., 2004, 34, 323–330 CrossRef CAS
.
- T. Kato, T. Inoue, T. Iwai and Y. Arai, J. Nucl. Mater., 2006, 357, 105–114 CrossRef CAS PubMed
.
- Y. Castrillejo, M. R. Bermejo, P. Diaz Arocas, F. de la Rosa and E. Barrado, Electrochemistry, 2005, 73, 636–643 CAS
.
- O. Shirai, M. Iizuka, T. Iwai and Y. Arai, Anal. Sci., 2001, 17, 51–57 CrossRef CAS
.
- P. Chamelot, L. Massot, L. Cassayre and P. Taxil, Electrochim. Acta, 2010, 55, 4758–4764 CrossRef CAS PubMed
.
- K. Liu, L. Y. Yuan, Y. L. Liu, X. L. Zhao, H. He, G. A. Ye, Z. F. Chai and W. Q. Shi, Electrochim. Acta, 2014, 130, 650–659 CrossRef CAS PubMed
.
- F. Lantelme, T. Cartaller, Y. Berghoute and M. Hamdani, J. Electrochem. Soc., 2001, 148, C604–C613 CrossRef CAS PubMed
.
- Y. Castrillejo, M. R. Bermejo, A. M. Martinez and P. Diaz Arcas, J. Min. Metall., Sect. B, 2003, 39, 109–135 CrossRef CAS
.
- P. Masset, R. J. M. Konings, R. Malmbeck, J. Serp and J. P. Glatz, J. Nucl. Mater., 2005, 344, 173–179 CrossRef CAS PubMed
.
- S. Vandarkuzhali, N. Gogoi, S. Ghosh, B. P. Reddy and K. Nagarajan, Electrochim. Acta, 2012, 59, 245–255 CrossRef CAS PubMed
.
- Y. L. Liu, L. Y. Yuan, G. A. Ye, K. Liu, L. Zhu, M. L. Zhang, Z. F. Chai and W. Q. Shi, Electrochim. Acta, 2014, 147, 104–113 CrossRef CAS PubMed
.
- H. Tang and B. Pesic, Electrochim. Acta, 2014, 119, 120–130 CrossRef CAS PubMed
.
- O. Shirai, A. Uehara, T. Fujii and H. Yamana, J. Nucl. Mater., 2005, 344, 142–145 CrossRef CAS PubMed
.
- J. Serp, P. Lefebvre, R. Malmbeck, J. Rebizant, P. Vallet and J. P. Glatz, J. Nucl. Mater., 2005, 340, 266–270 CrossRef CAS PubMed
.
- G. Cordoba and C. Caravaca, J. Electroanal. Chem., 2004, 572, 145–151 CrossRef CAS PubMed
.
-
A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2nd edn, 2001, p. 162 Search PubMed
.
- W. Sutherland, Philos. Mag., 1905, 9, 781–785 CrossRef CAS PubMed
.
-
Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermophysical and Electric Properties (Chapter 2), OECD Nuclear Energy Agency, 2007, p. 76 Search PubMed
.
- K. A. Gschneidner and F. W. Calderwood, Bull. Alloy Phase Diagrams, 1989, 10, 440–443 CrossRef CAS
.
- M. Li, Q. Q. Gu, W. Han, Y. D. Yan, M. L. Zhang, Y. Sun and W. Q. Shi, Electrochim. Acta, 2015, 167, 139–146 CrossRef CAS PubMed
.
- W. Weppner and R. A. Huggins, J. Electrochem. Soc., 1978, 125, 7–14 CrossRef CAS PubMed
.
- A. Morisson and C. Petot, Thermochim. Acta, 1987, 115, 167–173 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |