DOI:
10.1039/C5RA13565F
(Paper)
RSC Adv., 2015,
5, 77407-77416
Effects of post-treatment on the structure and properties of PVDF/FEP blend hollow fiber membranes†
Received
10th July 2015
, Accepted 2nd September 2015
First published on 3rd September 2015
Abstract
Polyvinylidene fluoride (PVDF)/poly(tetrafluoroethylene-co-hexafluo-ropropylene) (FEP) blend hollow fiber membranes were prepared by the melt-spinning method. The effects of drawing conditions (drawing ratio and temperature) on the structure and properties of the membranes were investigated by differential scanning calorimetry (DSC), dynamic mechanical analysis (DMA), and analysis of membrane morphology. The storage modulus increased with increasing drawing temperature at the same drawing ratio, which improved the membrane's elasticity. The quantity and pore size of the interfacial microvoids both increased significantly with an increase in the drawing ratio at the same drawing temperature. Finally, the membranes were evaluated for their performance in vacuum membrane distillation (VMD). All the membranes had a salt rejection higher than 99.0%.
1. Introduction
Poly(vinylidene fluoride) (PVDF) has excellent chemical resistance, physical and thermal stability, high strength, and flexibility that make it one of the most important polymeric membrane materials.1,2 However, PVDF membranes, utilized in membrane contactor (MC) processes, have a relatively weak hydrophobicity leading to undesired membrane wetting during MC processing. Due to the chemical structure of the perfluoro group, perfluoro-polymers, such as polytetrafluoroethylene (PTFE) and poly(tetrafluoroethylene-co-hexafluoropropylene) (FEP), are more hydrophobic than PVDF. Blending perfluoro-polymer with PVDF is therefore an effective way to enhance the hydrophobicity of PVDF membranes.3 Teoh et al.4 prepared PVDF/PTFE blend hollow fiber membranes by the phase inversion method. Incorporation of PTFE particles into the PVDF matrix enhanced the membrane hydrophobicity, yielding a resultant water contact angle of 103° when the PTFE loading was 50 wt%.
Compared to other hollow fiber membranes fabrication method, melt-spinning method have the advantage that causes little to no pollution (little or no solvents) and has low costs (little or no diluents, additives).5 However, the obtained hollow fiber membranes have low porosity and poor permeability that limit wide application. For membranes produced by melt-spinning method, post-treatment is frequently used to optimize performances.6–8 Drawing and heat-treatment affect membrane morphology and performances.9–15 A great number of studies have investigated the influences of post-treatment on membrane performance. Tabatabaei et al.16,17 studied the effects of drawing on polypropylene (PP) membrane and found that during hot drawing, the water vapor transmission rate improves with increasing the applied extension, and that the effect is inversed for cold drawing. Liu et al.18 fabricated polyurethane-based (PU-based) hollow fiber membranes by the melt-spinning method. The influence of hot-air treatment conditions, including theoretical drawing ratio, temperature, and time, on morphology and performance of membranes were investigated. The results show that the maximum mean pore size and pure water flux were obtained at a theoretical drawing ratio of 2 at 70 °C.
In this study, PVDF/FEP blend hollow fiber membranes were fabricated using the melt-spinning method. The polymer matrix phase was composed of PVDF while the dispersed phase was composed of micro-scale FEP particles. The effects of post-treatment (drawing ratio and temperature) on the blend hollow fiber membranes' morphology were then investigated. Finally, the performance of the PVDF/FEP blend hollow fiber membranes in vacuum membrane distillation (VMD) was studied.
2. Experimental
2.1 Materials
PVDF (Solef 6010, Solvay, Tm = 170 °C) was kindly provided by purchased from Solvay Co., Ltd, Belgium. Micro-scale FEP particles were purchased from Shandong Huaxiashenzhou Co., Ltd. The solvents N,N-dimethylacetamide (DMAc) and dioctyl phthalate (DOP) (Synthesis Grade, Tianjin Kermel Chemical Reagent Co., Ltd, >99.5%) were used diluted without further purification.
2.2 PVDF/FEP blend hollow fiber membrane preparation and treatment
Before use, the PVDF and FEP resins were dried for 12 h at 100 ± 2 °C in a vacuum oven (−1 bar). Then, at a particular mass ratio, PVDF, FEP, DMAc and DOP were homogeneously mixed under high speed agitation. Finally, the mixture was spun into hollow fiber membranes by the melt spinning method with a twin-screw spinning machine. The spinning conditions are reported in Table 1.
Table 1 Composition and spinning parameters of melt-spun hollow fiber membranes
| |
PVDF/FEP hollow fiber membrane |
| Membrane composition (PVDF/FEP/DMAc/DOP/Tween 80) (wt%) |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9:9 9:9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| Extrusion machine and spinneret temperature (°C) |
170.0 |
| Bore fluid |
N2 |
| Extra coagulation |
Water |
| Extra coagulation T (°C) |
25.0 ± 5.0 |
| Air gap (cm) |
15.0 |
| Extrusion speed (ml) |
14.0 |
| Take up speed (m min−1) |
30.7 |
| Spinneret dimension/mm |
OD/ID/L: 2.6/2.0/6.0 |
The post-treatments of PVDF/FEP blend hollow fiber membranes are described as follows: the PVDF/FEP blend hollow fiber membranes were placed in an electronic tensile testing machine (JBDL-200N) (Table 2). The blend hollow fiber membranes were stretched under a uniform speed at different drawing ratios (50, 100 and 150%), and under various drawing temperatures (25, 50, 90, and 150 °C). Drawing ratio (φ) was calculated according to eqn (1). Finally, the stretched hollow fiber membranes were fastened to maintain stretched length.
| |
 | (1) |
where
L2 and
L1 represent the length before and after post-treatment under tension, respectively.
Table 2 Sample designations and parameters of PVDF/FEP blend membranesa
| Drawing ratio (%) |
T (°C) |
| 25 |
50 |
90 |
130 |
| T106 represented that the drawing ratio (φ) was 106% under the condition of 25 °C. |
| 0 |
P-0 |
| 50 |
T5 |
F5 |
N5 |
H5 |
| 100 |
T10 |
F10 |
N10 |
H10 |
| 150 |
T106 |
F15 |
N15 |
H15 |
2.3 Membrane characterizations
2.3.1 Morphology observations. The morphologies of surface and cross-section of prepared hollow fiber membranes were observed using field emission scanning electron microscopy (FESEM, X4800; Hitachi, Japan). The membranes were immersed in liquid nitrogen for 10–15 s. Then the frozen membranes were broken for cross-section observation. The specimens were fixed on the conductive adhesive and coated with a thin layer of gold before observing.19
2.3.2 Porosity. The membrane porosity was defined as the pore volume divided by the total volume of the porous membrane. Membrane porosity was calculated by a gravimetric method, and was calculated according to eqn (2) below:20,21| |
 | (2) |
where, W1 is the weight of the wet membrane (g), W2 is the weight of the dry membrane (g), σ is the n-butanol density (ρ = 0.8098 g cm−3), D is the outer diameter (cm), d is the inner diameter (cm) and l is the length of sample membrane (cm).
2.3.3 Water contact angle. In order to evaluate the variations in the surface wetting characteristics of the PVDF/FEP blend hollow fiber membranes, the water contact angles of all the samples were measured using an optical contact angle meter (Jinshengxin Inspection instrument Co., Ltd, model JYSP-180). A water droplet was dropped on the sample surface, at least three water contact angles at different locations were recorded to get a reliable value, each sample was tested five times to evaluate the average value.
2.3.4 Permeability tests. The gas permeation flux of the dry membranes was determined by eqn (3). The permeate flow rate was measured under different pressures, as shown in Fig. 1. The lumen side of each membrane module was connected to a nitrogen cylinder. The permeation flux of nitrogen through the dried membranes was measured at room temperature.| |
 | (3) |
where J is the nitrogen flux (m3 m−2 h−1), L is the nitrogen flow (m3 h−1) and A is the membrane area (m2).
 |
| | Fig. 1 Gas permeation flux of the hollow fiber membrane testing device. | |
The liquid entry pressure (LEP) of water was measured to evaluate the membrane wetting resistance. LEP depends on pore size and hydrophobicity of the membrane. LEP was measured using a dead-end filtration set-up, which was designed according to the method described extensively by Smolders and Franken.22 The dry hydrophobic membrane was assessed at room temperature using a device made in the laboratory. The pressure of the feed side increased slowly in increments of 0.01 MPa during the measurement. At each pressure interval, the membrane was kept at a constant pressure for 5 min until the first permeate drop was obtained. The corresponding pressure value was considered the LEP point. Each sample was tested three times, and the values were averaged.
2.3.5 Thermal analysis. The dynamic thermal behavior of blend membranes was determined using dynamic mechanical analysis (DMA)(DMA242C, NETZSCH, Germany) between 0–300 °C at a heating rate of 5 °C min−1. The thermal properties of blend membranes were measured by differential scanning calorimetry (DSC)(DSC200F3, NETZSCH, Germany). DSC was performed at a heating rate of 10 °C min−1 over the range of 0–300 °C.
2.3.6 Membrane distillation (MD). Vacuum membrane distillation (VMD) was conducted to evaluate the permeate performance of PVDF/FEP blend hollow fiber membranes. The water desalination experiment was performed using a set-up schematically shown in Fig. 2. The shell side of the membrane was in contact with a hot, circulating NaCl aqueous solution (3.5 wt%) while its lumen side was connected to a vacuum pump to withdraw the permeated water vapor. The water vapor was subsequently condensed by a glass condenser using tap water as coolant. The condensed water was collected in a glass bottle, and its volume was determined with a measuring cylinder. The conductivity of the feed solution and permeate water were measured by a conductivity meter (AP-2, HM). The NaCl rejection R was calculated by eqn (4).| |
 | (4) |
where Cf and Cp were the conductivities of the feed solution and permeate water, respectively.
 |
| | Fig. 2 Schematic of vacuum membrane distillation (VMD) system (1-water bath; 2-magnetic circulating pump; 3 meter; 4-pressure gauge; 5-valve; 6-membrane component; 7-condenser pipe; 8-water flask; 9-vacuum pump). | |
3. Result and discussion
3.1 Membranes stress–strain curves
Effects of drawing temperature on the stress–strain curves of PVDF/FEP blend hollow fiber membranes are shown in Fig. 3. All the curves displayed a typical yield when the drawing ratio was 20–30%. While as the drawing temperature increased, the yield stress values reduced, and the yield point gradually weakened and eventually disappeared. With the increase in drawing temperature, polymer macromolecules have more movement, and therefore, the forces between molecules decrease. When the drawing temperature was lowered, the intermolecular force became larger, and the initial modulus of the PVDF/FEP blend hollow fiber membranebecamehigher. Meanwhile, the blend membranes' breaking strength and initial modulus increased monotonically with the decrease in drawing temperature. This may be due to the higher drawing temperature inducing a higher polymer chain activity, which resulted in a greater orientation of the hollow fiber membrane under the same stress.
 |
| | Fig. 3 Stress–strain curves of PVDF–FEP blend hollow fiber membranes. Drawing ratio: (a) 50%; (b) 100%; (c) 150%. | |
3.2 Formation mechanism of IFMs
Regarding the method to produce polymeric hollow fiber membranes, pore formation of the melt spinning method was much more difficult than solution spinning. Therefore, it was essential to improve the permeability of melt spun hollow fiber membranes. In our previous studies,18,23,24 nano-scale inorganic particles were introduced into the membrane polymer to create the IFMs by drawing. During the drawing process, IFMs formed between the polymer matrix and the dispersed phase of the inorganic particles. IFMs improved the porosity and permeability of hollow fiber membranes without reducing mechanical strength. In this study, the polymer particle of FEP was the dispersed phase, responsible for the strong hydrophilicity and the same C–F structure with PVDF. The formation mechanism of IFMs was shown in Fig. 4. Nascent PVDF/FEP hollow fiber membranes were composed of a continuous phase (PVDF) and a dispersed phase (FEP). To the best of our knowledge, the FEP particles kept a spherical shape. FEP had good rigidity under the spinning temperature of 170 °C, while the PVDF melted into fluid wrapping the FEP particles. When the blend hollow fiber membranes were drawn, there was an interface between the continuous phase and the dispersed phase due to separation. Furthermore, there were crazes in PVDF indicating damage to the amorphous regions during the drawing process.
 |
| | Fig. 4 The mechanism of IFM formation within drawn PVDF/FEP blend membranes. | |
3.3 Dynamic mechanical analysis
DMA curves of stretched PVDF/FEP blend hollow fiber membranes were shown in Fig. 5. The highest storage modulus (E′) was at a drawing ratio of 100% under the same drawing temperature (90 °C). When the drawing ratio was high enough (150%), an excess orientation was induced. The molecular arrangement had an excess orderliness, and the molecular interaction was too large. The elasticity of the blend hollow fiber membrane deteriorated. At the same drawing ratio, the storage modulus increased with the increase in drawing temperature. With increasing temperature, the material was more easily deformed. Along the direction of the stress, the polymer structure was in a more orderly arrangement, and there was an increase in the degree of crystallinity. During the drawing process, the ordered structure resulted in a larger rebound force, which was characterized by better elasticity.
 |
| | Fig. 5 DMA thermograms of post-treated PVDF/FEP blend hollow fiber membranes (a) E′ versus temperature; (b) tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ versus temperature. δ versus temperature. | |
As shown in Fig. 5(b), DMA was used to observe the relationship between tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and temperature. tan
δ and temperature. tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of a polymer was an important criterion for the compatibility of components.25,26 The tan
δ of a polymer was an important criterion for the compatibility of components.25,26 The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value of PVDF was about 170 °C, and that of FEP was about 275 °C. Analysis of PVDF/FEP blend hollow fiber membranes showed a peak near 180–200 °C and another near 250 °C. These peaks corresponded to the PVDF tan
δ value of PVDF was about 170 °C, and that of FEP was about 275 °C. Analysis of PVDF/FEP blend hollow fiber membranes showed a peak near 180–200 °C and another near 250 °C. These peaks corresponded to the PVDF tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak and the FEP tan
δ peak and the FEP tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak, respectively. When compared to the peaks of the two pure polymers, the tan
δ peak, respectively. When compared to the peaks of the two pure polymers, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak of PVDF in the blend moved to the right of that of the pure polymer, while the blend peak of FEP moved to the left of that of the pure polymer. The two tan
δ peak of PVDF in the blend moved to the right of that of the pure polymer, while the blend peak of FEP moved to the left of that of the pure polymer. The two tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peaks were close to each other, which indicated that PVDF and FEP were partially compatible in thermodynamics.
δ peaks were close to each other, which indicated that PVDF and FEP were partially compatible in thermodynamics.
3.4 Differential scanning calorimetry
DSC curves of the post-treated PVDF/FEP blend hollow fiber membranes under different drawing conditions were presented in Fig. 6. The melting peaks of the stretched PVDF/FEP blend hollow fiber membranes occurred around 172 °C and 260 °C corresponding to PVDF and FEP, respectively. This indicated that PVDF and FEP were physically mixed. The addition of FEP did not change the crystal structure of PVDF, and this was consistent with the DMA results. As shown in Fig. 6(a), the peak width of PVDF became wider at first and then became narrower. The peak area of PVDF at first decreased but then increased with increasing drawing ratios because the unstable crystalline phases were destroyed during the drawing process. With further drawing, the macromolecular chain orientation became regular, and this induced the improvement of the crystallinity. As can be seen from Fig. 6(b), under the same drawing ratio, there were no changes in the PVDF melting peak area, and the peak width became narrower with increasing drawing temperature. This might be because the macromolecular chains moved easier and arranged more regularly with an increase in drawing temperature. Also, some thin lamellae crystals were destroyed to form some thicker lamellae crystals which would have made crystallization more perfect.
 |
| | Fig. 6 DSC thermograms of post-treated PVDF/FEP blend hollow fiber membranes (a) varying drawing ratio; (b) varying drawing temperature. | |
3.5 Effect of the post-treatment on membrane's permeability
The permeability of PVDF/FEP blend hollow fiber membranes was characterized in terms of N2 flux as shown in Fig. 7. The N2 flux increased with an increasing drawing ratio. Increasing the drawing ratio resulted in higher porosity and IFMs' size, which further induced higher N2 flux. With regards to drawing temperature, the N2 flux first increased and then decreased as the drawing temperature increased at the same drawing ratio. The first increase of the N2 flux was explained by the same reason of the improvement of membrane porosity and IFMs' size when the drawing temperature was lower than 50 °C. To understand the decrease in the N2 flux, the glass transition temperature (Tg) of the FEP dispersed phase was approximately 90–95 °C. Therefore, as the drawing temperature rose to 90 °C, FEP polymer macromolecular chains also began to move, and FEP particles became deformed under tensile stress-elongation refinement. Some IFMs deformed into a long-narrow shape, and some even closed, inducing the decrease in pore size. In conclusion, the N2 flux of PVDF/FEP blend hollow fiber membranes first increased and then decreased with increasing temperature.
 |
| | Fig. 7 Effect of the post-treatment on N2 flux. | |
The trends of PVDF/FEP blend hollow fiber membranes' porosity by different post-treatments was shown in Fig. 8. The porosity of the P0 membrane was low. The porosity at first only slightly increased, and then significantly increased with an increase in drawing ratio. When the drawing ratio was low, increasing the temperature did not improve the porosity of the PVDF/FEP blend hollow fiber membranes. At low drawing ratios and increasing drawing temperature (above the Tg of FEP particles), both PVDF and FEP polymers deformed, the interface microvoids reduced, and porosity decreased. At large drawing ratios, the porosity of PVDF/FEP blend hollow fiber membranes significantly improved. Porosity increased because the tensile stress caused more IFMs in the blend hollow fiber membranes, and the tensile stress widened the original micropores. Under same drawing ratio, the porosity first decreased and then increased with increasing drawing temperature. The results agreed well with the permeability results as discussed above.
 |
| | Fig. 8 Effect of the post-treatment on porosity. | |
The effects of post-treatment on the LEP of PVDF/FEP blend hollow fiber membranes were illustrated in Fig. 9. For the hydrophobic membrane, the pore size and hydrophobicity dramatically affected the value of LEP. As shown in Fig. 9, with an increase in the drawing ratio, the LEP of PVDF/FEP blend hollow fiber membrane decreased and was more than 0.4 MPa. The decrease of LEP attributed to the size enhancement of IFMs, and this agreed very well with the analysis above.
 |
| | Fig. 9 Effect of the post-treatment on LEP. | |
In Fig. 10, the changes in the water contact angles of the post-treated PVDF/FEP blend hollow fiber membranes were displayed. The static contact angle of the P0 membrane was 116.3°. With an increase in the drawing ratio, more IFMs were produced in the PVDF/FEP blend hollow fiber membrane's surface, and therefore, the water was more likely to wet the surface of PVDF/FEP blend hollow fiber membrane as the static contact angle decreased.
 |
| | Fig. 10 Effect of the post-treatment on the water contact angle. | |
3.6 Effects of the post-treatment on membrane morphology
The outer surface morphologies of the post-treated PVDF/FEP blend hollow fiber membranes were shown in Fig. 11. The outer surface of the PVDF/FEP blend hollow fiber membrane P0, prepared by melt spinning method, was relatively dense. However, the outer surface was uneven from the FEP particles that were trapped within the PVDF polymer. After post-treatment, the outer surface of the PVDF/FEP blend hollow fiber membrane changed significantly. The outer surface's pores of the blend membrane became larger with increasing drawing ratio because PVDF and FEP were partially compatible. PVDF/FEP blend had a stress concentration region during the process of drawing. As well, the outer surface may have some defects, and when tensile stress was applied to the defects, irregular, elongated pores appeared at the surface along the drawing direction. The inner surface morphologies of the post-treated PVDF/FEP blend hollow fiber membranes were shown in Fig. 12. There were significant differences in morphology between the inner and the outer surfaces. The inner surface exhibited a much higher porosity than the outer surface. The porosity of the inner surface of the P0 membrane was less than that of the blend membrane. With an increase in the drawing ratio, the number of pores increased in the inner surface, and the pore sizes of post-treated blend membrane became larger. IFMs were clearly observed in the inner surface. When the temperature reached the Tg of FEP particles, the IFMs became more narrow and even partially closed when drawing ratio increased.
 |
| | Fig. 11 Effect of post-treatment on the outer surface morphology of PVDF/FEP blend membrane (drawing temperature: A-25 °C, B-50 °C, C-90 °C, D-130 °C, drawing ratio: 1–50%, 2–100%, 3–150%). | |
 |
| | Fig. 12 Effect of post-treatment on the inner surface morphology of PVDF/FEP blend membrane (drawing temperature: A-25 °C, B-50 °C, C-90 °C, D-130 °C, drawing ratio: 1–50%, 2–100%, 3–150%). | |
The cross-section morphologies of the post-treated PVDF/FEP blend hollow fiber membranes were shown in Fig. 13(A) large number of FEP particles were dispersed in the polymer membrane. The P0 membrane's cross-section was dense and exhibited a much lower porosity. With an increase in the drawing ratio, the number of pores increased while the pore sizes became larger.
 |
| | Fig. 13 Effect of post-treatment on the cross-section morphology of PVDF/FEP blend membrane (drawing temperature: A-25 °C, B-50 °C, C-90 °C, D-130 °C, drawing ratio: 1–50%, 2–100%, 3–150%). | |
3.7 Membrane distillation performance
The post-treated PVDF/FEP blend hollow fiber membranes were tested for the VMD process, and permeate flux results were shown in Fig. 14. The maximum permeate flux of the N15 membrane reached as high as 3.2 L m−2 h−1. Moreover, with an increasing drawing ratio, permeate flux increased due to the increased number and pore sizes of IFMs. Thus, membrane distillation (MD) flux had an obvious growth trend as the drawing ratio increased.
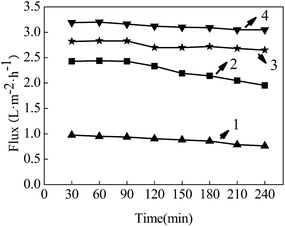 |
| | Fig. 14 Influence of post-treatment on the PVDF/FEP blend hollow fiber membrane distillation flux, 1-raw, 2-N5, 3-N10, 4-N15. | |
The salt rejection of a post-treated PVDF/FEP blend hollow fiber membrane module at an operating temperature of 70 °C was shown in Fig. 15. All PVDF/FEP blend hollow fiber membrane desalination rates were higher than 99% among the different post-treatment conditions. The P0 membrane desalination rate was significantly higher than that of post-treated PVDF/FEP blend hollow fiber membrane, and the desalination rates were relatively stable.
 |
| | Fig. 15 The effect of post-treatment on the PVDF/FEP blend hollow fiber membrane component of desalting. | |
4. Conclusions
PVDF/FEP blend hollow fiber membranes were fabricated by the melt-spinning method. Effects of drawing ratio and temperature on the structure and the performances of PVDF/FEP blend hollow fiber membranes were investigated. Increasing the drawing ratio induced an increase in both the pore number and size of the outer surface. The porosity of the PVDF/FEP blend membrane was significantly promoted while the LEP decreased as the drawing ratio increased. It was concluded that the PVDF and FEP were partially compatible systems from the results of DSC and DMA analysis.
Appendix
Abbreviation
| φ | Drawing ratio % |
| l | Length of membrane m |
| ε | Porosity % |
| W | Weight of membrane g |
| D | Outer diameter cm |
| d | Inner diameter cm |
| J | Nitrogen flux m3 m−2 h−1 |
| R | NaCl rejection % |
| Cp | Conductivities of the feed solution μs cm−1 |
| Cf | Conductivities of the permeate water μs cm−1 |
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21404079, 51402212), Tianjin Key Research Program of Application Foundation and Advanced Technology (No. 12JCZDJC26600).
References
- J. E. Dohany and L. E. Robb, Polyvinylidene fluoride, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, New York, 3rd edn, 1980, vol. 11, p. 64 Search PubMed.
- A. J. Lovinger, Poly(vinylidene fluoride), in Development in Crystalline Polymers, ed. D. C. Bassett, Appl. Sci., 1982, vol. 1, p. 195 Search PubMed.
- G.-D. Kang and Y.-M. Cao, Application and modification of poly(vinylidene fluoride) (PVDF) membranes, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS PubMed.
- M. M. Teoh and T. S. Chung, Membrane distillation with hydrophobic macrovoid-free PVDF-PTFE hollow fiber membranes, Sep. Purif. Technol., 2009, 66, 229–236 CrossRef CAS PubMed.
- J. Kim, S. S. Kim, M. Park and M. Jang, Effects of precursor properties on the preparation of polyethylene hollow fiber membranes by stretching, J. Membr. Sci., 2008, 318, 201–209 CrossRef CAS PubMed.
- J. Lee, H. K. Lee and K. E. Rasmussen, et al., Environmental and bioanalytical applications of hollow fiber membrane liquid-phase microextraction: a review, Anal. Chim. Acta, 2008, 624, 253–268 CrossRef CAS PubMed.
- K. Song, Y. Zhang and J. Meng, et al., Structural polymer-based carbon nanotube composite fibers: understanding the processing–structure–performance relationship, Materials, 2013, 6, 2543–2577 CrossRef CAS PubMed.
- C. Zhao, X. Zhou and Y. Yue, Determination of pore size and pore size distribution on the surface of hollow-fiber filtration membranes: a review of methods, Desalination, 2000, 129, 107–123 CrossRef CAS.
- I. C. Kim, H. G. Yun and K. H. Lee, Preparation of asymmetric polyacrylonitrile membrane with small pore size by phase inversion and post-treatment process, J. Membr. Sci., 2002, 199, 75–84 CrossRef CAS.
- L. S. Wan, Z. K. Xu, X. J. Huang, A. F. Che and Z. G. Wang, A novel process for the post-treatment of polyacrylonitrile-based membranes: performance improvement and possible mechanism, J. Membr. Sci., 2006, 277, 157–164 CrossRef CAS PubMed.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, Microporous membranes obtained from polypropylene blend films by stretching, J. Membr. Sci., 2008, 325, 772–782 CrossRef CAS PubMed.
- M. T. Liu, C. F. Xiao and X. Y. Hu, Optimization of polyurethane-based hollow fiber membranes morphology and performance by post-treatment methods, Desalination, 2011, 275, 133–140 CrossRef CAS PubMed.
- H. L. Liu, C. F. Xiao, X. Y. Hu and M. T. Liu, Post-treatment effect on morphology and performance of polyurethane-based hollow fiber membranes through melt-spinning method, J. Membr. Sci., 2013, 427, 326–335 CrossRef CAS PubMed.
- J. J. Kim, T. S. Jang, Y. D. Kwon, U. Y. Kim and S. S. Kim, Structural study of microporous polypropylene hollow fiber membranes made by the melt-spinning and cold stretching method, J. Membr. Sci., 1994, 93, 209–215 CrossRef CAS.
- S. Y. Lee, S. Y. Park and H. S. Song, Lamellar crystalline structure of hard elastic HDPE films and its influence on microporous membrane formation, Polymer, 2006, 47, 3540–3547 CrossRef CAS PubMed.
- S. H. Tabatabaei, P. J. Carreau and A. Ajji, Microporous membranes obtained from polypropylene blend films by stretching, J. Membr. Sci., 2008, 325, 772–782 CrossRef CAS PubMed.
- A. Rahimpour, et al., The effect of heat treatment of PES and PVDF ultrafiltration membranes on morphology and performance for milk filtration, J. Membr. Sci., 2009, 330, 189–204 CrossRef CAS PubMed.
- H. Liu, C. Xiao, X. Hu and M. Liu, Post-treatment effect on morphology and performance of polyurethane-based hollow fiber membranes through melt-spinning method, J. Membr. Sci., 2013, 427, 326–335 CrossRef CAS PubMed.
- H. A. Tsai, et al., Heat-treatment effect on the morphology and pervaporation performances of asymmetric PAN hollow fiber membranes, J. Membr. Sci., 2005, 255(1–2), 33–47 CrossRef CAS PubMed.
- L. F. Han, Z. L. Xu, Y. Cao, Y. M. Wei and H. T. Xu, Preparation, characterization and permeation property of Al2O3, Al2O3-SiO2 and Al2O3-kaolin hollowfiber membranes, J. Membr. Sci., 2011, 372, 154–164 CrossRef CAS PubMed.
- X. F. Li, Y. G. Wang, X. L. Lu and C. F. Xiao, Morphology changes of polyvinylidene fluoride membrane under different phase separation mechanisms, J. Membr. Sci., 2008, 320, 477–482 CrossRef CAS PubMed.
- K. Smolders and A. C. M. Franken, Terminology for membrane distillation, Desalination, 1989, 72, 249–262 CrossRef CAS.
- Q. Huang, C. Xiao, X. Hu and S. An, Fabrication and properties of poly(tetrafluoroethylene-co-hexafluoropropylene) hollow fiber membranes, J. Mater. Chem., 2011, 21, 16510–16516 RSC.
- M. Liu, C. Xiao and X. Hu, Optimization of polyurethane-based hollow fiber membranes morphology and performance by post-treatment methods, Desalination, 2011, 275, 133–140 CrossRef CAS PubMed.
- S. Zeng, C. Reyes and J. Liu, et al. Facile hydroxylation of halloysite nanotubes for epoxy nanocomposite applications, Polymer, 2014, 55, 6519–6528 CrossRef CAS PubMed.
- K. Song, Y. Zhang and M. L. Minus, Polymer Interphase Self-Reinforcement and Strengthening Mechanisms in Low-Loaded Nanocomposite Fibers, Macromol. Chem. Phys., 2015, 216, 1313–1320 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13565f |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9:9
9:9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ versus temperature.
δ versus temperature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and temperature. tan
δ and temperature. tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of a polymer was an important criterion for the compatibility of components.25,26 The tan
δ of a polymer was an important criterion for the compatibility of components.25,26 The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value of PVDF was about 170 °C, and that of FEP was about 275 °C. Analysis of PVDF/FEP blend hollow fiber membranes showed a peak near 180–200 °C and another near 250 °C. These peaks corresponded to the PVDF tan
δ value of PVDF was about 170 °C, and that of FEP was about 275 °C. Analysis of PVDF/FEP blend hollow fiber membranes showed a peak near 180–200 °C and another near 250 °C. These peaks corresponded to the PVDF tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak and the FEP tan
δ peak and the FEP tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak, respectively. When compared to the peaks of the two pure polymers, the tan
δ peak, respectively. When compared to the peaks of the two pure polymers, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak of PVDF in the blend moved to the right of that of the pure polymer, while the blend peak of FEP moved to the left of that of the pure polymer. The two tan
δ peak of PVDF in the blend moved to the right of that of the pure polymer, while the blend peak of FEP moved to the left of that of the pure polymer. The two tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peaks were close to each other, which indicated that PVDF and FEP were partially compatible in thermodynamics.
δ peaks were close to each other, which indicated that PVDF and FEP were partially compatible in thermodynamics.
















