Biotic oxidation of polyethylene using a bio-surfactant produced by B. licheniformis: a novel technique
Shritama Mukherjeea,
Uttam Ray Chaudhurib and
Patit P. Kundu*a
aAdvanced Polymer Laboratory, Department of Polymer Science and Technology, University of Calcutta, 92 APC Road, Kolkata-9, West Bengal, India. E-mail: ppk923@yahoo.com; Tel: +91 3323525106
bChemical Technology, University of Calcutta, 92 APC Road, Kolkata-9, West Bengal, India
First published on 20th August 2015
Abstract
Polyethylene was incubated with a bio-surfactant producing bacterium B. licheniformis for 2 months in a suitable media. Low concentrations of NaCl were added to study its effect on the bio-surfactants activity. Being amphiphilic, the surfactant has a unique ability to decrease the surface energy, and this decrease measured using the surface tension of the medium was 50%. The surfactant was able to oxidize both control (unoxidized) and pre-oxidized polyethylene during incubation. The oxidation level of the control polyethylene sample was increased in the presence of NaCl, and the oxidation level was higher in the presence of 1% NaCl as compared to that found with 0.5% NaCl. A higher amount of surfactant was also produced, as observed by the comparatively low surface tension in the presence of NaCl. During the bio-oxidation of polyethylene, higher amounts of unsaturated hydrocarbons were formed as compared to carbonyl groups. This oxidation was also observed through the reduced crystalline property and cracked polyethylene surface using SEM. It was also observed that the product formed during the oxidation by the bio-surfactant could be solubilised into liquid media. For this rapid loss of oxidation product, a decrease in the mechanical properties of all the treated polyethylene samples was observed, and this deterioration was highest in the case of the pre-oxidised polyethylene incubated with the bio-surfactant for 2 months. In this study, a novel unique method for the bio-oxidation of polyethylene using a bio-surfactant was established.
1. Introduction
The use of polyethylene films has increased immensely over the last decade for their low cost, strong mechanical properties with relatively low thickness, multiple chemical resistance and longevity. Due to the absence of any polar groups in the closely packed carbon–hydrogen backbone and its high molecular weight, polyethylene is resistant towards abiotic oxidation and microbial attack under natural conditions. Therefore, polyethylene once produced, does not degrade naturally, resulting in a high amount of waste polyethylene accumulation (25 million tons per year) in the environment. This environmental pollution has become one of the most important concerns for researchers and lots of studies have been carried out to find a way for the degradation of polyethylene waste.1,2 Decades of research have shown that biotic, i.e. microbial, degradation of polyethylene is the only method for its environmental friendly degradation. Microbial degradation is mainly achieved by the formation of a bio-film on the polyethylene surface. Many microbes, i.e. Lysinibacillus xylanilyticus, Aspergillus niger, Penicillium pinophilum, and Rhodococcus ruber, have been used for the microbial degradation of polyethylene.1,3,4 To enhance microbial attachment to the polyethylene surface, the hydrophobic properties of polyethylene should be changed into hydrophilic ones through abiotic oxidation.1 UV irradiation has been used in several studies for the abiotic oxidation of polyethylene by introducing polar groups into the polymer backbone, which thereby reduces the hydrophobic properties; for example, polyethylene containing a pro-oxidant has been oxidised using UV light for 60 h with an increase in the carbonyl index.1,3,5 In another study, polyethylene mixed with pro-oxidant additives has been oxidised using UV light, and the highest oxidation level was achieved after 600 h.2 Another way to increase the degradation rate, other than using a pre-oxidation step, is by increasing the contact surface between polyethylene and water by adding a surfactant in the biodegradation system.Surfactants are wide varieties of surface active amphiphilic molecules (both hydrophilic and hydrophobic regions are present in the same molecule).6 Surfactants have an ability to reduce the surface tension and interfacial tension of a solution. The bioavailability of non-soluble/hydrophobic material is enhanced by the reduction of the surface tension. Surfactants also have the ability to enhance the solubility of petroleum hydrocarbons. Surface tension reduction ability of surfactants can be used to increase the biodegradation of polyethylene. In some of the polyethylene biodegradation studies, a chemical surfactant has been used in the system. Increased biodegradation of pre-oxidised polyethylene has been reported in soil after the addition of a surfactant into the system.7 In another study, enhanced bio-film formation has been observed by adding Tween 80 into a polyethylene biodegradation system containing Pseudomonas aeruginosa.8 Another type of surfactant known as bio-surfactant is produced by different microbes.9 The main advantages of bio-surfactants over chemical surfactants are their environmental friendly nature and their bio-degradability. One such bio-surfactant producing bacterium is Pseudomonas aeruginosa and its bio-surfactant is called rhamnolipid.10,11 This specific bacterium has been used to study the biodegradation of polypropylene, and bio-film formation was enhanced by rhamnolipid production.12 The bio-surfactant producing bacteria, Bacillus pumilus, B. halodenitrificans, and B. cereus, have been reported for the biodegradation of pre-oxidised polyethylene containing a pro-oxidant with enhanced biodegradation.2 There are mainly 6 types of bio-surfactants produced by bacteria; among these, lipopeptides are most important and effective bio-surfactants for high surface activity, which are mainly produced by different strains of Bacillus.6,13 Such a bio-surfactant producing bacterium is Bacillus licheniformis, which is isolated from oil reservoirs.14 This bacterium has also been used to enhance oil recovery through the production of a biosurfactant.5,15,16 The bio-surfactant produced by this bacterium is known as lichenysin and is a lipopeptide, which has been reported for its ability to reduce the surface tension.16 B. licheniformis can be used for changing the hydrophobic properties of polyethylene into hydrophilic ones upon the action of the bio-surfactant produced by this bacterium in the presence of polyethylene. For this treatment, a suitable growth medium should be used for optimum production and optimum activity of the bio-surfactant. Previously, it is reported that a higher amount of bio-surfactant is produced by B. licheniformis in a growth medium without any trace of NaCl compared to a medium containing 0.5% NaCl.17 However, in another study, 5% NaCl concentration in a growth medium is reported as the optimum condition for the maximum production of the bio-surfactant.16
Bio-surfactant producing Bacillus licheniformis has not yet been used to study its effect on the oxidation of polyethylene. The oxidation of unoxidized polyethylene and the chemical modification of pre-oxidized polyethylene by the bio-surfactant produced using B. licheniformis have been reported in this study in the presence or absence of NaCl during the incubation of polyethylene for 2 months.
2. Materials and methods
2.1. Test materials
Daily used 0.01 mm thick, transparent colourless polyethylene bags were collected from the waste bins of Kolkata Municipal Corporation. The bags were then cut into rectangular pieces (5 mm × 5 mm) and washed vigorously with soap water and distilled water to remove any debris and bio-material attached to the polyethylene surface. The rectangular pieces were then dried at 60 °C overnight in a hot-air oven. These unoxidised polyethylene films were used as the control polyethylene films.For heat-UV light treatment, the rectangular pieces of polyethylene films were taken into a beaker and placed in a custom-made chamber, where the temperature was maintained at 60 °C under continuous UV light for 1 month. The wavelength of the UV light used was within the range of 350–200 nm.
2.2. Microbial culture
Bacillus licheniformis JF2 (ATCC no. 39307, MTCC no. 2454) was used for bio-treatment study. This microbial culture was obtained from the Institute of Microbial Technology, Chandigarh, India. The microbial culture was maintained in a nutrient broth (Himedia). The bio-treatment was carried out in an YPD medium containing 10 g of yeast extract, 20 g of glucose and 20 g of peptone in 1 litre of double distilled H2O at 37 °C for different time periods. 0.5% and 1% sodium chloride (NaCl) solutions were added to an YPD medium incubated with B. licheniformis containing the control polyethylene to study its effect on the stability of the bio-surfactant and its ability to reduce the surface tension. The control polyethylene samples incubated with B. licheniformis in the YPD growth medium without NaCl and with NaCl at 1% and 0.5% concentration and UV-light-treated polyethylene films incubated with B. licheniformis in a YPD growth medium were kept for 2 months. After 1 and 2 months, samples from each case were harvested, washed and dried. For negative control, polyethylene samples were placed in an YPD growth medium without any bacterial species. All the samples were incubated at 37 °C and in triplicate.The surface tension (σ) of the microbial culture medium was measured using a stalagmometer at 25 °C at day zero and at different intervals of time.18 The surface tension was calculated using the following formula.
2.3. Characterization of polyethylene
FTIR analysis was carried out using an ATR-FTIR (model alpha, Bruker, Germany) spectrometer, scanning from 4000 cm−1 to 500 cm−1 at room temperature. The resolution was set at 4 cm−1 with 42 scans per spectrum. The carbonyl index (C.I.) and double bond index (D.B.I.) were calculated using the ratio of the absorbance frequency of the carbonyl peak (1740 cm−1) and double bond (1650 cm−1) to that of the CH2 group bending frequency (1465 cm−1), respectively.All the polyethylene samples were sputter coated with a gold layer using a Hitachi sputter coater (model-E1010 Ion Sputter), Japan. Photomicrographs were observed under a scanning electron microscope (EVO 18, Carl Zeiss, Germany).
X-ray diffraction studies for all the types of polyethylene samples were carried out with an X-ray diffractometer (PANalytical, Netherlands) in the 2θ angle range from 3° to 50° with a fixed scan rate of 1° min−1. The percentage (%) of crystallinity was calculated using the following formula.
All the samples were observed under AFM (Bruker-Germany, model: Innova) without carrying out any type of pre-treatment. Depth analysis of the surface of the treated polyethylene was carried out using ‘Nanoscape analysis’ software.
Tensile properties of all the polyethylene samples were investigated using a tensile tester (Tinius Olsen H5KT, ASTM D638 standard) at 23 °C with a cross head speed of 20 mm min−1. Eight samples were cut into rectangular shapes of 100 mm in length, 10 mm in width and 0.01 mm in thickness.
3. Results and discussion
3.1. Surface tension
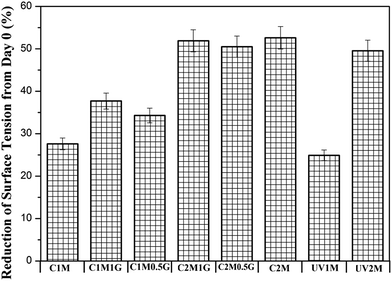
Reduction in the surface tension from day 0 in percentage is presented in Fig. 1. Minimum surface tension is achieved after 2 months of incubation with B. licheniformis with both control and pre-oxidized polyethylene with or without NaCl. The surface tension reduction from day 0, after 2 months of incubation with B. licheniformis was 51.9% in the case of YPD medium with control polyethylene and 1% NaCl solution; 50.5% in the case of YPD medium with control polyethylene and 0.5% of NaCl; 52.6% in the case of YPD medium with control polyethylene and without NaCl and 49.6% in the case of YPD medium with UVPE. After 1 month, minimum surface tension was achieved in the case of YPD medium incubated with B. licheniformis in the presence of 1% of NaCl and control polyethylene. The presence of surface active molecules can be indirectly predicted from this reduction in surface tension. In a previous study, surface tension reduction from 70 mN m−1 to 58.8 mN m−1 in the presence of a pro-oxidant containing polyethylene using B. pumilus, B. halodenitrificans and B. cereus was reported.2 However, in the present study, the minimum surface tension achieved in the presence of control polyethylene (unoxidised) and UVPE using B. licheniformis is considerably lower than the previously reported ones. NaCl was added in the growth medium to observe its effect on the surface tension reduction ability of the bio-surfactant. It is clear from the abovementioned results that after 1 month, the lowest surface tension is observed in the case of the growth medium containing 1% NaCl. As reported in previous studies, lower concentrations of NaCl stabilize the bio-surfactant and increase its activity; this may be the reason for the comparatively lower surface tension observed for the bio-surfactant produced using B. licheniformis in the presence of 1% NaCl.16 After 2 months, a minimum surface tension was achieved, which was slightly higher than the minimum surface tension reported using B. licheniformis and is almost same in all the cases of YPD medium incubated with B. licheniformis with control and pre-oxidized polyethylene with or without any NaCl.16 Thus, it can be inferred that the presence of NaCl does not affect the surface tension reduction ability of the bio-surfactant after 2 months of incubation, though the presence of NaCl stabilizes the bio-surfactant produced using B. licheniformis in the presence of polyethylene after 1 month of incubation. In the case of the negative control, i.e. polyethylene kept in the YPD medium without any bacteria, no change in the surface tension was observed during 2 months of bio-treatment (Fig. 13).3.2. Characterization of polyethylene incubated with B. lichenformis
Control polyethylene films were incubated with B. licheniformis for 1 (C1M) and 2 (C2M) months in an YPD medium without NaCl. The control polyethylene samples were also incubated with B. licheniformis in an YPD medium containing 1% (C1M1G) and 0.5% (C1M0.5G) of NaCl for 1 month and 2 months (C2M1G for 1% NaCl and C2M0.5G for 0.5% NaCl). To study the effect of the surfactant on the pre-oxidized polyethylene sample, polyethylene samples oxidized using UV light were also incubated with B. licheniformis for 1 (UV1M) and 2 (UV2M) months in an YPD medium. Polyethylene incubated in an YPD growth medium without any bacterial species was also characterized. No bio-film formation was observed on the treated polyethylene samples incubated with B. licheniformis.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–)). After 2 months of incubation of B. licheniformis in an YPD medium, in the case of C2M1G (1% of NaCl), C2M0.5G (0.5% of NaCl) and C2M (without NaCl), the peak intensity at 1660 cm−1 (for unsaturated hydrocarbons) increases as compared to those found for C1M1G, C1M0.5G and C2M, respectively. In the case of C2M, the peak intensity at 1740 cm−1 (for ketones) increases slightly as compared to C1M; in the case of C2M1G and C2M0.5G, the peak intensity at 1740 cm−1 (for ketones) decreases as compared to that for C1M1G and C1M0.5G, respectively (Fig. 2). This indicates that more unsaturated hydrocarbons are formed as compared to the ketone groups. In case of the UV-treated PE (UVPE) (Fig. 3), after 1 and 2 months, the intensity of the peak in the 1800–1500 cm−1 region increases. In the case of UV1M, an increase in the intensity of the peak at 1740 cm−1 is observed due to the formation of ketones. However, after 2 months, the intensity of the peak at 1740 cm−1 decreases and the intensity of the peak at 1660 cm−1 increases. The carbonyl index (C.I.) and double bond index (D.B.I.) of the treated polyethylene films were compared with those found for untreated polyethylene, i.e. control PE and UVPE, as shown in Fig. 4. Both the C.I. and D.B.I. of the treated polyethylene films are observed to increase compared to that found for the untreated polyethylene (control and UVPE) films. The formation of unsaturated hydrocarbons is considerably higher than that of the C
C–)). After 2 months of incubation of B. licheniformis in an YPD medium, in the case of C2M1G (1% of NaCl), C2M0.5G (0.5% of NaCl) and C2M (without NaCl), the peak intensity at 1660 cm−1 (for unsaturated hydrocarbons) increases as compared to those found for C1M1G, C1M0.5G and C2M, respectively. In the case of C2M, the peak intensity at 1740 cm−1 (for ketones) increases slightly as compared to C1M; in the case of C2M1G and C2M0.5G, the peak intensity at 1740 cm−1 (for ketones) decreases as compared to that for C1M1G and C1M0.5G, respectively (Fig. 2). This indicates that more unsaturated hydrocarbons are formed as compared to the ketone groups. In case of the UV-treated PE (UVPE) (Fig. 3), after 1 and 2 months, the intensity of the peak in the 1800–1500 cm−1 region increases. In the case of UV1M, an increase in the intensity of the peak at 1740 cm−1 is observed due to the formation of ketones. However, after 2 months, the intensity of the peak at 1740 cm−1 decreases and the intensity of the peak at 1660 cm−1 increases. The carbonyl index (C.I.) and double bond index (D.B.I.) of the treated polyethylene films were compared with those found for untreated polyethylene, i.e. control PE and UVPE, as shown in Fig. 4. Both the C.I. and D.B.I. of the treated polyethylene films are observed to increase compared to that found for the untreated polyethylene (control and UVPE) films. The formation of unsaturated hydrocarbons is considerably higher than that of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in case of all the treated control polyethylene, except for C1M1G, as observed from the FTIR spectra and C.I.–D.B.I. graph (Fig. 2 and 4). However, after 2 months, an increase in the D.B.I. is observed in the case of C2M1G, C2M0.5G and C2M. In the case of UVPE, after 1 month, the formation of C
O bonds in case of all the treated control polyethylene, except for C1M1G, as observed from the FTIR spectra and C.I.–D.B.I. graph (Fig. 2 and 4). However, after 2 months, an increase in the D.B.I. is observed in the case of C2M1G, C2M0.5G and C2M. In the case of UVPE, after 1 month, the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups is comparatively higher than the formation of unsaturated hydrocarbons. However, after 2 months, in the case of UV2M, the amount of C
O groups is comparatively higher than the formation of unsaturated hydrocarbons. However, after 2 months, in the case of UV2M, the amount of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups is comparatively lower than the amount of unsaturated hydrocarbons. From this result, it is evident that the polar groups are formed on the polymer backbone. After oxidation using UV light and heat treatment, polyethylene shows similar changes, which were observed through an increased carbonyl index.1 Because bio-surfactant is an amphiphilic molecule, it has the ability to increase the solubilisation of hydrocarbons. The hydrophobic part of the bio-surfactant remains attached with the polyethylene surface, while the hydrophilic part protrudes towards the aqueous solution. This phenomenon enhances the polyethylene's availability to dissolved oxygen, which further results in the oxidation of polyethylene. In case of the pre-oxidized polyethylene samples, the oxidation rate is higher than that found for the control polyethylene. The previously formed oxidation product present in the pre-oxidized polyethylene may assist the oxidation reaction initiated by the bio-surfactant. However, after 2 months, the already formed oxidation product may get solubilised into the aqueous medium, resulting in a decrease in the intensity of the peak at 1740 cm−1. Another reason for this phenomenon can be the conversion of carbonyl groups into double bonds due to its further oxidation. A similar phenomenon is also reported in another study, where the conversion of carbonyl groups into double bonds was observed during the degradation process using Lysinibacillus sp. In this study, 42% reduction in the carbonyl index and 200% increase in the double bond index is reported after 18 weeks of incubation with Lysinibacillus sp. with the UV irradiated films.3 However, this reduction in the carbonyl index is 32% and the increase in the double bond index is 85% in the case of UVPE incubated in the presence of the bio-surfactant for 2 months, which is considerably higher than the previously reported data. In another study, 75% reduction in the carbonyl index of pre-oxidized polyethylene containing a pro-oxidant using a bacterial strain in 21 days was reported. The formation of unsaturated hydrocarbons after bacterial treatment is also reported in GC-MS studies.2
O groups is comparatively lower than the amount of unsaturated hydrocarbons. From this result, it is evident that the polar groups are formed on the polymer backbone. After oxidation using UV light and heat treatment, polyethylene shows similar changes, which were observed through an increased carbonyl index.1 Because bio-surfactant is an amphiphilic molecule, it has the ability to increase the solubilisation of hydrocarbons. The hydrophobic part of the bio-surfactant remains attached with the polyethylene surface, while the hydrophilic part protrudes towards the aqueous solution. This phenomenon enhances the polyethylene's availability to dissolved oxygen, which further results in the oxidation of polyethylene. In case of the pre-oxidized polyethylene samples, the oxidation rate is higher than that found for the control polyethylene. The previously formed oxidation product present in the pre-oxidized polyethylene may assist the oxidation reaction initiated by the bio-surfactant. However, after 2 months, the already formed oxidation product may get solubilised into the aqueous medium, resulting in a decrease in the intensity of the peak at 1740 cm−1. Another reason for this phenomenon can be the conversion of carbonyl groups into double bonds due to its further oxidation. A similar phenomenon is also reported in another study, where the conversion of carbonyl groups into double bonds was observed during the degradation process using Lysinibacillus sp. In this study, 42% reduction in the carbonyl index and 200% increase in the double bond index is reported after 18 weeks of incubation with Lysinibacillus sp. with the UV irradiated films.3 However, this reduction in the carbonyl index is 32% and the increase in the double bond index is 85% in the case of UVPE incubated in the presence of the bio-surfactant for 2 months, which is considerably higher than the previously reported data. In another study, 75% reduction in the carbonyl index of pre-oxidized polyethylene containing a pro-oxidant using a bacterial strain in 21 days was reported. The formation of unsaturated hydrocarbons after bacterial treatment is also reported in GC-MS studies.2
 | ||
| Fig. 2 FTIR spectra of treated control (unoxidized) polyethylene. A – control PE, B – C1M; C – C1M1G; D – C1M0.5G; E – C2M1G; F – C2M0.5G; G – C2M; and H – negative control. | ||
No change in the peak intensity was observed in the case of the negative control from the untreated control polyethylene.
 | ||
| Fig. 5 XRD spectra of A – control PE, B – C1M, C – C1M1G, D – C1M0.5G, E – C2M, F – C2M1G, and G – C2M0.5G. | ||
3.2.3.1. Scanning electron microscopy. Surface morphology of the biologically treated and untreated control polyethylene samples was observed using SEM, and the images are presented in Fig. 8. Images of surface morphology for the biologically treated and untreated UVPE are presented in Fig. 9. Surface morphology of all the biologically treated polyethylene samples is rough and severely cracked. This type of crack formation on the surface is usually observed in the case of oxidized polyethylene samples that result from natural contractions. The cracks are formed due to cross-linking during oxidation or due to the loss of oxidation product by solubilisation from the surface of polyethylene into the bacterial medium.2,3,24
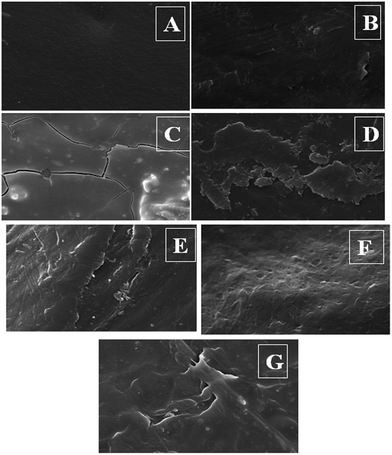 | ||
| Fig. 8 SEM images: (A) control PE, (B) C1M, (C) C1M1G, (D) C1M0.5G, (E) C2M, (F) C2M1G, and (G) C2M0.5G. | ||
3.2.3.2. Atomic force microscopy. Contact mode AFM height images of the biologically treated and untreated polyethylene samples are presented in Fig. 10. The AFM graph shows the amount and depth of cavities formed during the treatment of the polyethylene surface. The depth of the cavity formed during the biological treatment increases and more cavities form on the surface of the polyethylene in the case of C1M sample. This cavity formation process becomes slower after 1 month. After 2 months of biological treatment, deeper cavities are formed on the surface of C2M but the amount is considerably less than that found on C1M. As it is already reported that the amorphous region is readily available for oxidation than the crystalline region of the semi-crystalline polyethylene molecule, these cavities are formed due to the formation of nodules that are made up of slightly or completely unoxidized crystalline parts of polyethylene and due to the solubilisation of the oxidation product into the liquid media; this is caused due to the oxidation of the amorphous region by the bio-surfactant.18,24 During 1 month of biological treatment, in the case of C1M, the amorphous region is oxidised. After 2 months, the oxidation process proceeds further, oxidizing the amorphous region. However, in the case of C2M, the depth of the cavity increases rather than the amount of cavities formed on the polyethylene surface. Moreover, C2M is oxidized more than it is solubilised, which may be the reason for the slight increase in the depth of the cavity as compared to C1M. The depth of the cavity is more in the case of C1M0.5G than that found for C1M1G. In the case of C1M1G, both the amorphous region and crystalline region are oxidised, from where the oxidation product solubilises into the liquid medium, forming less deep cavities. This can also be inferred from the reduced crystallinity levels obtained using the XRD data (Fig. 7). Therefore, the depth of the cavities is less in the case of C1M1G. In the case of C1M0.5G, only the amorphous region is oxidised, leaving the crystalline region very slightly or completely unoxidized; this creates nodules on the surface with deeper cavities. This result is also in accordance with the observed crystallinity level shown in Fig. 7. In the case of C2M0.5G, this slightly or completely unoxidized crystalline part of C1M0.5G is further oxidized during 2 months of bacterial incubation. Reduction in the crystallinity of C2M0.5G is may be because of this phenomenon. The crystallinity (%) of C1M0.5G is slightly lower than the untreated control polyethylene. Although in both the C2M and C1M0.5G cases, only the amorphous region is oxidised, but deeper cavities are formed in the case of C1M0.5G because the oxidation level was higher in the case of C1M0.5G than in the case of C2M. In the case of UV2M, after 2 months, due to the loss of oxidation product from the amorphous and crystalline regions, the cavity depth was considerably less than the other treated polyethylene samples. After 1 month, both the crystalline and amorphous regions were oxidised, as observed through the reduced crystallinity levels shown in Fig. 7. After 2 months, in the case of UV2M, the oxidation product, formed due to UV light treatment during 1 month of incubation of UVPE with surfactant, solubilised into the liquid medium, resulting in lesser deep cavities. This phenomenon can be co-related with the slightly increased crystallinity levels.
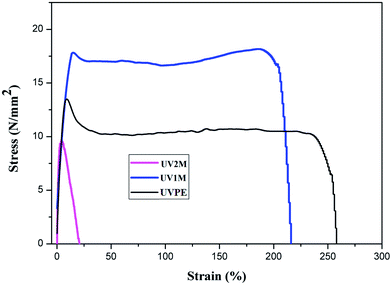
The stress versus strain graphs of the treated and untreated UVPE are plotted in Fig. 12. After 1 month of biological treatment, the elongation at break of UV1M decreases. However, this decrease in the elongation at break is major in the case of UV2M. This polyethylene sample shows total loss in tensile properties. The higher oxidation rate and structural modification can be the reasons for the loss in tensile properties. The oxidation products formed during UV light treatment and during two months of bacterial incubation dissolve into the liquid media, as observed through SEM and in depth analysis by AFM; this is another reason for the loss of the mechanical properties.
The negative control polyethylene sample, kept in an YPD medium without any bacterial species, does not show any changes in the chemical, physical or mechanical properties during 2 months of bio-treatment (Fig. 13).
Polyethylene is oxidized in the presence of the bio-surfactant produced by B. licheniformis. In the presence of NaCl, the oxidation level is higher. During 2 months of incubation, the already formed oxidation products get solubilised into the aqueous medium, which is a characteristic property of the surfactant. This property is also enhanced in the presence of NaCl. Therefore, NaCl present in the medium can stabilise and enhance the activity of the bio-surfactant as reported earlier. A similar level of oxidation of the polyethylene films in a high density form is reported by Ojeda et al. after natural weathering for 161 days; this can be achieved in 60 days using a bio-surfactant initiated oxidation process.26 Natural weathering or accelerated weathering is mostly used for the oxidation of polyethylene. If polyethylene is mixed with pro-oxidant additives, then the oxidation process via this method is very effective and fast. However, in the case of polyethylene films without a pro-oxidant or commercial polyethylene with an added antioxidant, oxidation using this process can take 9 months to 1 year. Although a pro-oxidant initiates the process of oxidation rapidly, this process is not much economical. On the other hand, bio-surfactants are environmental friendly and more effective than the abovementioned processes. In addition, they can be easily isolated after polyethylene treatment and can be used for any other application. In addition, the solubilisation of the oxidation products into an aqueous medium can further result into a degradation process with weight loss if carried out for a long duration.
4. Conclusion
Continuous production of a bio-surfactant using B. licheniformis in the presence of polyethylene proved to be an effective process for the oxidation of polyethylene. The presence of a low concentration of NaCl in the YPD growth medium not only stabilized the bio-surfactant produced but also enhanced its activity, as observed through the higher level of oxidation of the polyethylene in the presence of a low concentration of NaCl. In the case of pre-oxidized polyethylene, oxidization and solubilisation of the oxidation products into an aqueous medium was also observed.Acknowledgements
Shritama Mukherjee is grateful to Council for Scientific and Industrial Research (CSIR), New Delhi, for providing senior research fellowship to conduct this research. Authors thank DBT, IPLS, for providing AFM imaging service.References
- I. Gilan, Y. Hadar and A. Sivan, Appl. Microbiol. Biotechnol., 2004, 65(1), 97–104 CAS.
- P. K. Roy, S. Titus, P. Surekha, E. Tulsi, C. Deshmukh and C. Rajagopal, Polym. Degrad. Stab., 2008, 93(10), 1917–1922 CrossRef CAS PubMed.
- A. Esmaeili, A. A. Pourbabaee, A. H. Alikhani, F. Shabani and E. Esmaeili, PLoS One, 2013, 8(9), 71720 Search PubMed.
- V. T. Sepulveda, G. S. Castaneda, M. G. Rojas, A. Manjur and E. F. Torres, J. Appl. Polym. Sci., 2002, 83(2), 305–314 CrossRef PubMed.
- B. Lee, A. L. Pometto, A. Fratzke and T. B. Bailey Jr, Appl. Environ. Microbiol., 1991, 57, 678 CAS.
- S.-C. Lin, M. A. Minton, M. M. Sharma and G. Georgioui, Appl. Environ. Microbiol., 1994, 60(1), 31–38 CAS.
- S. Karlsson, O. Ljungquist and A.-C. Albertsson, Polym. Degrad. Stab., 1988, 21, 237–250 CrossRef CAS.
- A.-C. Albertsson, C. Sares and S. Karlsson, Acta Polym., 1993, 44, 243–246 CrossRef CAS PubMed.
- C. N. Mulligan, Environ. Pollut., 2005, 133, 183–198 CrossRef CAS PubMed.
- L. Guerra-Santos, O. Kappeli and A. Fiechter, Pseudomonas, Appl. Environ. Microbiol., 1984, 48(2), 301–305 CAS.
- R. M. Maier and G. Soberon-Chavez, Appl. Microbiol. Biotechnol., 2000, 54, 625–633 CrossRef CAS.
- J. Arutchelvi, C. Joseph and M. Doble, Biochem. Eng. J., 2011, 56, 37–45 CrossRef CAS PubMed.
- H. Ghojavand, F. Vahabzadeh, E. Roayaei and A. K. Shahrak, J. Colloid Interface Sci., 2008, 324, 172–176 CrossRef CAS PubMed.
- G. E. Jeneman, M. J. McInerney and R. M. Knapp, et al., Dev. Ind. Microbiol., 1983, 24, 485–492 Search PubMed.
- M. Javaheri, G. E. Jenneman, M. J. McInerney and R. M. Knapp, Appl. Environ. Microbiol., 1985, 50(3), 698–700 CAS.
- M. M. Yakimov, K. N. Timmis, V. Wray and H. L. Fredrickson, Appl. Environ. Microbiol., 1995, 61(5), 1706–1713 CAS.
- S. C. Lin, M. M. Sharma and G. Georgiou, Biotechnol. Prog., 1993, 9, 138–145 CrossRef CAS.
- D. Konz, S. Doekel and M. A. Marahie, J. Bacteriol., 1999, 181(1), 133–140 CAS.
- M. S. RabelloTand and J. R. White, Polymer, 1997, 38(26), 6389–6399 CrossRef.
- F. Khabbaz, A. C. Albertsson and S. Karlsson, Polym. Degrad. Stab., 1999, 63, 127–138 CrossRef CAS.
- A. Corti, S. Muniyasamy, M. Vitali, H. S. Imam and E. Chiellini, Polym. Degrad. Stab., 2010, 95, 1106–1114 CrossRef CAS PubMed.
- A. Benitez, J. J. Sánchez, L. M. Arnal, J. A. Müller, O. Rodríguez and G. Morales, Polym. Degrad. Stab., 2013, 98, 490–501 CrossRef CAS PubMed.
- M. El-Awady and M. Natural, J. Appl. Polym. Sci., 2003, 87, 2365–2371 CrossRef CAS PubMed.
- N. Medard, J.-C. Soutif and F. Poncin-Epaillard, Surf. Coat. Technol., 2002, 160, 197–205 CrossRef CAS.
- V. Švorčík, K. Kolářová, P. Slepička, A. Mackova, M. Novotna and V. Hnatowicz, Polym. Degrad. Stab., 2006, 91, 1219–1225 CrossRef PubMed.
- T. Ojeda, A. Freitas, K. Birck, E. Dalmolin, R. Jacques, F. Bento and F. Camargo, Polym. Degrad. Stab., 2011, 96, 703–707 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |