Improving nutraceutical bioavailability using mixed colloidal delivery systems: lipid nanoparticles increase tangeretin bioaccessibility and absorption from tangeretin-loaded zein nanoparticles
Abstract
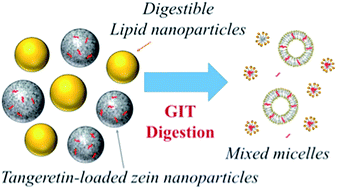
The objective of this study was to evaluate the influence of dietary lipids on the gastrointestinal fate of tangeretin-loaded zein nanoparticles. The zein delivery systems were mixed with different amounts of oil-in-water emulsions to represent varying levels of digestible fat in the diet, and then passed through a simulated gastrointestinal tract model. Tangeretin bioaccessibility increased with increasing fat content due to enhanced solubilization within the mixed micelles formed by lipid digestion products (fatty acids and monoacylglycerols) in the intestinal fluids. Indeed, the tangeretin concentration in the micelle phase was about twelve times higher in the presence of fat droplets than in their absence. The intestinal epithelium absorption study indicated that tangeretin permeability across the model epithelium cells also increased with increasing fat content. This study suggests that utilizing mixed colloidal systems consisting of both lipid nanoparticles and protein nanoparticles may promote the bioavailability of hydrophobic bioactive agents.

 Please wait while we load your content...
Please wait while we load your content...