An electrochemistry assisted approach for fast, low-cost and gram-scale synthesis of mesoporous silica nanoparticles†
Abstract
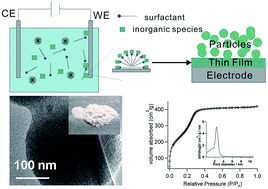
We report a facile electrochemistry assisted sol–gel approach for the preparation of mesoporous silica nanoparticles (MSNs) at the gram scale on common conductive substrates, such as the stainless steel surface. The formation of MSNs was triggered by electrochemical generation of hydroxide at the substrate/solution interface, which induced the self-assembly of surfactant micelles and meanwhile catalysed the polycondensation of silica precursors. The as-prepared MSNs were characterized in detail by several instrumental analysis techniques, revealing the obtained MSNs have a uniform pore size of about 2.4 nm and a high surface area of about 1164 m2 g−1. We believe that this approach can provide a simple, fast and low-cost way to produce MSNs and may be potentially useful for the large-scale industrial production.

 Please wait while we load your content...
Please wait while we load your content...