A polydiacetylene-based fluorescence assay for the measurement of lipid membrane affinity
Abstract
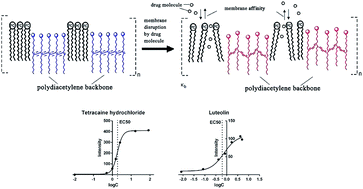
Polydiacetylene (PDA) is a promising membrane-screening tool because lipid constituents can be incorporated into the PDA framework to form lipid/PDA vesicles used as lipid bilayers. Previous reports have shown that the colorimetric signals of PDA could be utilized for the measurement of drug–lipid membrane interactions. In this study, the fluorescence signals of PDA vesicles were investigated for the measurement of lipid membrane affinity. Based on the fluorescence response of PDA vesicles (excitation wavelength 485 nm, emission wavelength 560 nm), the half maximal response concentration (EC50) was introduced for the evaluation of drug–membrane interactions. In order to validate this method, local anesthetics and flavonoids were selected as the reference compounds and their log(EC50) values correlated well with other lipid membrane affinity constants. Then the influence of buffer pH conditions and lipid constituents on the membrane affinity were investigated to show the wide application of this method using tetracaine hydrochloride as the reference compound. The particle size of vesicles before and after addition of tetracaine hydrochloride was determined to observe the extent of vesicle binding of the tested compound. The zeta potential results showed that the electrostatic interaction had less effect on the change of lipid membrane affinity at different pH value. Therefore, the hydrophobic interaction was assumed to play the most important role in the increase in lipid membrane affinity of tetracaine hydrochloride as the buffer pH value increased. The ratio of Chol in the lipid constituents affected the affinity of tetracaine hydrochloride less, but significantly weakened the sensitivity of PDA-based fluorescence signals. In summary, this work provides a simple, sensitive and reproducible PDA-based fluorescent method for the rapid measurement of lipid membrane affinity.

 Please wait while we load your content...
Please wait while we load your content...