A new rhodamine based chemodosimeter for Ni2+ with high sensitivity and selectivity†
Ivan Zhanga,
Yi Wanga,
Chao Wanb,
Zhen Xingc,
Wen Lia,
Minjie Li*a and
Sean Xiao-An Zhang*a
aState Key Lab of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, People's Republic of China. E-mail: liminjie@jlu.edu.cn; seanzhang@jlu.edu.cn; Fax: +86-431-85153812
bCollege of Environment and Resources, Jilin University, People's Republic of China
cCollege of Life of Science, Jilin University, People's Republic of China
First published on 30th July 2015
Abstract
A new rhodamine derivative by modifying the ortho-position of the carboxylate in benzolate with amino pyridine has been developed, which exhibits high sensitivity and selectivity toward Ni2+ with a detection limit down to 4.6 ppb.
Nickel (Ni) is widely used in various industrial applications such as in electroplating, Ni–Cd batteries, pigments for paints, ceramics, catalysts for hydrogenation, surgical and dental prostheses and as magnetic tapes for computers. Besides, the Ni2+ is an essential cofactor for a variety of enzymes that play important roles in microorganisms and plants, particularly in energy and nitrogen metabolism. However, excessive Ni2+ in our body will result in adverse health effects ranging from allergic dermatitis to lung and nasal sinus cancers.1 Therefore, the detection of Ni2+ is very important. Up to now, various methods have been recently reported for the determination of Ni2+ concentration in different biological, industrial and food samples. The mature methods to detect Ni2+ are mostly based on time consuming and sophisticated analytical techniques, such as atomic absorption/emission spectrometry, liquid chromatography and voltammetry.2 Although these methods are accurate, they are not suitable for convenient “in-the-field” detection as they normally require expensive instruments and sample pretreatment, and also have serious influence by the interference of coexisting ions. Therefore, it is very important to develop sensitive, rapid, and simple-to-use methods to sense Ni2+. Meanwhile, colorimetric sensors have also attracted much attention, because the detection can be analyzed by the naked eye. It also allows on-site and real-time detection in an uncomplicated and inexpensive manner, providing qualitative and quantitative information.3 Unfortunately, colorimetric sensors for Ni2+ with high selectivity and sensitivity are rare.4
Rhodamine B is a widely used organic dye due to their excellent photophysical properties, such as high extinction coefficients, excellent quantum yields, and relatively long emission wavelengths.5 But the partial tautomerization from lactone form to zwitterionic form in polar solvent leads to the strong background signal of rhodamine B, which limits its application in chemosensors (Fig. 1a and S1, ESI†).6 But since Czarnik and the co-worker reported the first modified-rhodamine for detecting Cu2+ in 1997, many rhodamine-based probes have been developed.7 However, the modification in these probes are usually on 2′ position (Rhodamine 2′, e.g., the oxygen atom is replaced by nitrogen atom or sulfur atom) and they can only detect some common cations (such as Cu2+, Hg2+, Fe3+, Cr3+ and so on) (Fig. 1b left).8 To our best knowledge, the highly sensitive and selective rhodamine-based probe for Ni2+ hasn't been developed yet. The probable reason is that the present Rhodamine 2′ could not well chelate Ni2+ and some new modifications on the skeleton of rhodamine seem particularly important. Here, we put forward a new modification strategy on rhodamine by introducing some proper ligands to ortho-position of carboxylate group in rhodamine (Rhodamine 3′, Fig. 1b right) and they would exhibit several advantages: (1) Y atom and R group in the molecular skeleton will be quite variable and they could be expected to detect different analytes by suitable combinations of Y atom and R group; (2) this modification could well maintain carboxylate group to participate in the chelation and finally enhance the recognition ability. As a prototype, we designed and synthesized a new ortho-modified rhodamine derivative of Rha-py (Fig. 1c). As we expected, the residual carboxylate group participates in the chelation of Ni2+ with other ligands and finally realizes the detection of Ni2+ with high selectivity and sensitivity.
Rha-py was facilely synthesized in high yield with only three steps. Detailed synthesis was given in ESI.† We first tested the optical properties of Rha-py in aqueous solution. Rha-py showed a strong absorption at 550 nm in pure water (Fig. S1†), indicating that Rha-py partially existed in zwitterion form.9 And the fluorescence of the probe was weak, which is presumably caused by the effect of photo-induced electron transfer (PET) from adjacent electron-donating amine on its benzyl carboxylate structure.10 In the meanwhile, the thermal dissipation of excitation energy of the probing molecule by strong H bonding with water is another reason for the weak fluorescence. Because water has both the high polarity and H-bond donating and accepting properties, which will effectively decrease capability of coordination from its three built-in functional groups of amine, pyridine and carboxylate, metal ions are not easy to be fixed by these coordination groups in pure water.
Therefore, further evaluation of the Rha-py was then conducted in various mixed solutions of water and CH3CN, and results indicate that a non-negligible absorption for ring-opened form of Rha-py still exist in neutral condition if the percentage of CH3CN in solution is less than 50%. CH3CN–H2O (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is finally selected as an ideal test solvent to avoid the background absorption (Fig. S1, ESI†). The absorbance at 550 nm was about 40-fold enhanced in pH range from 4.5 to 7.5 (in HEPES buffer) in presence of 10 equivalents Ni2+ (Fig. S2, ESI†), showing obvious pink color from colorless solution. Detailed sensing capability of this probe toward various cations were systematically investigated thereafter with UV-Vis spectroscopy in CH3CN–H2O solution (v/v = 9
1) is finally selected as an ideal test solvent to avoid the background absorption (Fig. S1, ESI†). The absorbance at 550 nm was about 40-fold enhanced in pH range from 4.5 to 7.5 (in HEPES buffer) in presence of 10 equivalents Ni2+ (Fig. S2, ESI†), showing obvious pink color from colorless solution. Detailed sensing capability of this probe toward various cations were systematically investigated thereafter with UV-Vis spectroscopy in CH3CN–H2O solution (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with HEPES buffer at pH = 7.1.
1) with HEPES buffer at pH = 7.1.
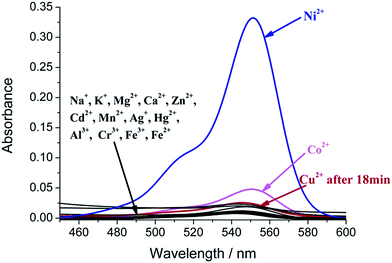
Tested cations include Na+, K+, Mg2+, Ca2+, Zn2+, Co2+, Ni2+, Cu2+, Cd2+, Mn2+, Ag+, Hg2+, Al3+, Cr3+, Fe3+, Fe2+ and the counter ions are NO3− or Cl−. When 10 equivalents of these cations (c = 40 μM) were added to the test solution of Rha-py (c = 4 μM) at room temperature, the solutions remained colorless respectively, except for the Ni2+, Co2+ and Cu2+. Upon addition of Ni2+, a dramatic pink color was observed by the naked eye immediately. In corresponding UV-Vis spectra, a new absorption band peaked at about 550 nm was formed herein, which is in good agreement with its color change on Ni2+ (Fig. 2). Furthermore, the pink color from this Ni2+ sensing still remained after a day. Co2+ also resulted in a very weak increment in absorption due possible to the similarity in size with Ni2+. Although, Cu2+ also induced ring-opening reaction initially with the appearance of pink color, the pink color however disappeared quickly in ten minutes (Fig. 3), but the pink color kept for a rather long time in presence of Ni2+ (Fig. S8, ESI†). In order to find out the reason, LC-MS experiments were carried out (Fig. S11, ESI†), the retention time of Rha-py was 1.8 min with its mass signal at 549.3, and the retention time of Rha-amino (amino group on the 3′ position of rhodamine B) was 2.7 min with its mass signal at 458.2. However, in the mixture of Cu2+ and Rha-py, no signal of Rha-py or its metal complex with Cu2+ was found, but the signal of Rha-amino. We presumed Rha-py could coordinate with Cu2+ well, but Cu2+ will catalyze its degradation to generate Rha-amino. Rha-amino cannot fully coordinate with Cu2+ and it exists in colorless lactone form.11 This distinct difference on color fading time will help to differentiate these two interfering cations in practical sensing. In corresponding fluorescence spectra, Ni2+ induced about 5-fold enhancement (Fig. S3, ESI†), and Cu2+ quenched the fluorescence of the probe, and we presumed it is because their electron configuration of d-orbital and the coordinating effect are different.
An important feature of a sensor is its selectivity toward the analyte relative to other competitive species. Therefore, competition experiments were carried out by adding Ni2+ (c = 40 μM) to a solution of Rha-py (c = 4 μM) in the presence of miscellaneous cations (c = 40 μM), respectively. These miscellaneous competitive ions did not induce significant absorption changes of Rha-py in the absence of Ni2+, except Cu2+, which caused the color change at first and then the color faded in minutes. Upon addition of Ni2+ to the above solutions, pink color appeared except for the one with Cu2+ in it, which didn't show further color change (Fig. 4). These results revealed that Rha-py had a remarkable selectivity toward Ni2+ with the only interference of Cu2+, however, by monitoring the time-resolved spectra or color fading rate, Cu2+ can be differentiated.
To identify the stoichiometry between Ni2+ and Rha-py, Job's plot had been drawn (Fig. S4, ESI†). We can find when the molar fraction of Ni2+ was 0.5, the absorbance at 550 nm reached a maximum, indicating the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between Rha-py and Ni2+. And the association constant (Ka) of Rha-py with Ni2+ was determined as 4.87 × 104 M−1 using the Benesi–Hildebrand equation (Fig. S6, ESI†).12a The spectroscopic detection limit for Ni2+ was 7.8 × 10−8 M (Fig. S7, ESI†), calculated with the equation of 3σ/S.12b Which is lower than the upper limit 0.04 mg L−1 (6.8 × 10−7 M) recommended by the U. S. Environmental Protection Agency (EPA) for drinking water.13
1 complex between Rha-py and Ni2+. And the association constant (Ka) of Rha-py with Ni2+ was determined as 4.87 × 104 M−1 using the Benesi–Hildebrand equation (Fig. S6, ESI†).12a The spectroscopic detection limit for Ni2+ was 7.8 × 10−8 M (Fig. S7, ESI†), calculated with the equation of 3σ/S.12b Which is lower than the upper limit 0.04 mg L−1 (6.8 × 10−7 M) recommended by the U. S. Environmental Protection Agency (EPA) for drinking water.13
The interaction mechanism of Ni2+ with Rha-py was further studied with infrared spectroscopy (IR) and MALDI-TOF experiments. From the IR spectra (Fig. S9, ESI†), the stretching vibration absorption peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and vibration of pyridine have a significant shift towards lower wavenumbers, and the vibration of carboxyl acid appeared, indicating the complexation with Ni2+.14 In MALDI-TOF, the number 605.34 stands for the complex of Ni2+ and Rha-py, which confirmed the coordination ratio was 1
O and vibration of pyridine have a significant shift towards lower wavenumbers, and the vibration of carboxyl acid appeared, indicating the complexation with Ni2+.14 In MALDI-TOF, the number 605.34 stands for the complex of Ni2+ and Rha-py, which confirmed the coordination ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S10, ESI†).
1 (Fig. S10, ESI†).
In order to test the practicality of the Rha-py probe, we took the water from South Lake, Changchun as a sample. The sample was filtered to remove organisms and analyzed by the proposed absorption method under optimized conditions, and no Ni2+ is detected. Then Ni2+ is added to the sample on purpose. The recovery of Ni2+ and R.S.D. of probe Rha-py are satisfactory (Table S1 ESI†),15 indicating that the present colorimetric probe of Rha-py was applicable for the determination of Ni2+ in contaminated water samples.
Conclusions
In summary, we first demonstrated the modification strategy on the 3′ position of rhodamine and developed a colorimetric probe for Ni2+. The prototype probe of Rha-py based on the strategy shows high sensitivity and selectivity for Ni2+. The probe could respond to Ni2+ quickly. Unfortunately, Cu2+ is an interference for Ni2+ detection, however, it can be differentiated by time-resolved spectra or color fading rate. The spectroscopic detection limit is lower than the upper limit recommended by EPA for drinking water. With good performance in analysis of Ni2+ in lake water, we believe that this novel Ni2+ chemosensor can be used for monitoring Ni2+ in contaminated water samples with high sensitivity and selectivity. This work will be a starting point of further research, we will make further effort to modify the 3′ position along with the modification on the other position. This will surely inspire more development of chemosensors for environmental and medical applications.Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 51373068) and program for Chang Jiang Scholars and Innovative Research Team in University (No. IRT101713018).Notes and references
- (a) P. H. Kuck, Mineral Commodity Summaries: Nickel, United States Geological Survey, 2006 Search PubMed; (b) J. R. Davis, Uses of Nickel, ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys, ASM International, 2000, p. 7 Search PubMed; (c) E. Denkhaus and K. Salnikow, Crit. Rev. Oncol. Hematol., 2002, 42, 35 CrossRef CAS; (d) M. E. Rothenberg, Nat. Immunol., 2010, 11, 781 CrossRef CAS PubMed; (e) D. P. Giedroc and A. I. Arunkumar, Dalton Trans., 2007, 3107 RSC.
- (a) S. Carter, A. S. Fisher, M. W. Hinds and S. Lancaster, J. Anal. At. Spectrom., 2012, 27, 2003 RSC; (b) V. K. Gupta, R. N. Goyal, S. Agarwal, P. Kumar and N. Bachheti, Talanta, 2007, 71, 795 CrossRef CAS PubMed; (c) V. K. Gupta, R. Prasad, P. Kumar and R. Mangla, Anal. Chim. Acta, 2000, 420, 19 CrossRef CAS; (d) V. K. Gupta, A. K. Singh and M. K. Pal, Anal. Chim. Acta, 2008, 624, 223 CrossRef CAS PubMed.
- (a) D. Xiong and H. Li, Nanotechnology, 2008, 19, 465502 CrossRef PubMed; (b) H. Zhu, J. Fan, B. Wang and X. Peng, Chem. Soc. Rev., 2015, 44, 4337 RSC; (c) X. Lou, L. Zhang, J. Qin and Z. Li, Chem. Commun., 2008, 5848 RSC; (d) X. M. Liu, Q. Zhao, Y. Li, W. C. Song, Y. P. Li, Z. Chang and X. H. Bu, Chin. Chem. Lett., 2013, 24, 962 CrossRef CAS PubMed.
- (a) K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed; (b) N. Kaur and S. Kumar, Chem. Commun., 2007, 3069 RSC; (c) S. Goswami, S. Chakraborty, A. K. Das, A. Manna, A. Bhattacharyya, C. K. Quah and H. K. Fu, RSC Adv., 2014, 4, 20922 RSC; (d) F. A. Abebe, C. S. Eribal, G. Ramakrishna and E. Sinn, Tetrahedron Lett., 2011, 52, 5554 CrossRef CAS PubMed.
- (a) M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC; (b) M. J. Uddin and L. J. Marnett, Org. Lett., 2008, 10, 4799 CrossRef CAS PubMed; (c) T. D. Horvath, G. Kim, R. Kopelman and S. Ashkenazi, Analyst, 2008, 133, 747 RSC; (d) F. Yan, L. Chen, X. Yan, F. Ge and E. Duan, Progr. Chem., 2006, 18, 252 CAS.
- (a) X. Yang, Y. Guo and R. M. Strongin, Org. Biomol. Chem., 2012, 10, 2739 RSC; (b) Y. K. Yang, K. J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS PubMed; (c) K. M. K. Swamy, S. K. Ko, S. K. Kwon, H. N. Lee, C. Mao, J. M. Kim, K.-H. Lee, J. H. Kim, I. Shin and J. Yoon, Chem. Commun., 2008, 5915 RSC; (d) A. J. Weerasinghe, F. A. Abebe and E. Sinn, Tetrahedron Lett., 2011, 52, 5648 CrossRef CAS PubMed.
- (a) V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS; (b) A. J. Weerasinghe, C. Schmiesing, S. Varaganti, G. Ramakrishna and E. Sinn, J. Phys. Chem. B, 2010, 114, 9413 CrossRef CAS PubMed; (c) H. Zheng, X. Q. Zhan, Q. N. Bian and X. J. Zhang, Chem. Commun., 2013, 49, 429 RSC; (d) B. Qiao, S. G. Sun, N. Jiang, S. Zhang and X. J. Peng, Dalton Trans., 2014, 4626 RSC; (e) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Y. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- (a) A. J. Weerasinghe, C. Schmiesing and E. Sinn, Tetrahedron Lett., 2009, 50, 6407 CrossRef CAS PubMed; (b) E. M. Nolan and S. J. Lippard, J. Am. Chem. Soc., 2007, 129, 5910 CrossRef CAS PubMed; (c) X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025 CrossRef CAS PubMed; (d) Y. Xiang and A. Tong, Org. Lett., 2006, 8, 1549 CrossRef CAS PubMed; (e) Y. K. Yang, K. J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS PubMed; (f) Z. G. Zhou, M. X. Yu, H. Yang, K. W. Huang, F. Y. Li, T. Yi and C. H. Huang, Chem. Commun., 2008, 3387 RSC; (g) Y. Koide, Y. Urano, S. Kenmoku, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 10324 CrossRef CAS PubMed; (h) X. D. Lou, Y. Zhang, Q. Q. Li, J. G. Qin and Z. Li, Chem. Commun., 2011, 47, 3189 RSC; (i) S. Wen and H. M. Ma, Chem. Commun., 2008, 16, 1856 Search PubMed; (j) K. B. Li, Y. Zang, H. Wang, J. Li, G. R. Chen, T. D. James, X. P. He and H. Tian, Chem. Commun., 2014, 50, 11735 RSC; (k) Z. X. Han, B. S. Zhu, T. L. Wu, Q. Q. Yang, Y. L. Xue, Z. Zhang and X. Y. Wu, Chin. Chem. Lett., 2014, 25, 73 CrossRef CAS PubMed.
- N. V. Abrosimova, A. G. Stepanova, I. A. Krasavin and B. V. Parusnikov, J. Appl. Spectrosc., 1988, 48, 610 CrossRef.
- (a) C. Munkholm, D. R. Parkinson and D. R. Walt, J. Am. Chem. Soc., 1990, 112, 2608 CrossRef CAS; (b) A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385 RSC.
- M. Taki, S. Iyoshi, A. Ojida, I. Hamachi and Y. Yamamoto, J. Am. Chem. Soc., 2010, 132, 5938 CrossRef CAS PubMed.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS; (b) IUPAC Commission on Spectrochemical and Other Optical Procedures for Analysis, Pure Appl. Chem., 1976, 45, 107 Search PubMed.
- G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433 CrossRef CAS PubMed.
- (a) G. R. Woodburn, N. R. Lien, A. Hoppe, Y. Jahng and J. R. Telford, J. Coord. Chem., 2010, 63, 185 CrossRef CAS PubMed; (b) X. Liu, Q. Lin, T.-B. Wei and Y.-M. Zhang, New J. Chem., 2014, 38, 1418 RSC.
- M. Wang, F.-Y. Yan, Y. Zou, N. Yang, L. Chen and L.-G. Chen, Spectrochim. Acta, Part A, 2014, 123, 216 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1HNMR, 13CNMR data, LC-HRMS data, X-ray diffraction data, synthetic details and spectra. CCDC 1036431. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra11737b |
| This journal is © The Royal Society of Chemistry 2015 |