The effects of P. aeruginosa ATCC 9027 and NTA on phytoextraction of Cd by ramie (Boehmeria nivea (L.) Gaud)
Jieli Xieab,
Yunguo Liu*ab,
Guangming Zengab,
Huan Liuab,
Bohong Zhengc,
Hui Tangab,
Weihua Xuab,
Zhichao Sunab,
Xiaofei Tanab,
Jian Nieab,
Zhengjiang Jiangab,
Chao Ganab and
Shufan Wangab
aCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: xjlhnu@163.com; Fax: +86 731 88822829; Tel: +86 731 88649208
bKey Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, P. R. China
cSchool of Architecture and Art Central South University, Central South University, Changsha 410082, P. R. China
First published on 31st July 2015
Abstract
In pot experiments, the effects of Pseudomonas aeruginosa ATCC 9027 and nitrilotriacetic acid (NTA) on Cd phytoextraction from contaminated soil by Boehmeria nivea (L.) Gaud (ramie) was investigated. Ramie was grown in a sandy soil in the presence of 30 mg kg−1 Cd and 50 mg kg−1 Cd, respectively. The experimental pots were amended with P. aeruginosa ATCC 9027 or NTA at different levels (5, 10 and 20 mmol kg−1) weekly. The results showed that the inoculation of P. aeruginosa ATCC 9027 alleviated the Cd-induced damages, resulting in promotion of ramie growth, improvement of antioxidative enzymes activities and increase of total Cd-uptake by ramie. By contrasting 30 and 50 mg kg−1 Cd treatments, the inoculation of P. aeruginosa ATCC 9027 increased accumulation in the roots ranging from 54% to 96% and 13% to 104% in 30 and 50 mg kg−1 Cd soils, respectively. The average accumulation of Cd with P. aeruginosa ATCC 9027 was about 1.95-fold (30 mg kg−1 Cd) and 1.54-fold (50 mg kg−1 Cd) compared to the corresponding NTA treatments. When added with NTA, the accumulation of Cd in the shoots of ramie was higher than the controls, but inhibition of plant growth and related enzyme activities were observed. The experimental results demonstrated that P. aeruginosa ATCC 9027 can greatly enhance phytoremediation efficiency. Besides, the results also indicated that P. aeruginosa ATCC 9027 was more effective than NTA to improve the efficiency of ramie under cadmium stress in practical applications.
1. Introduction
Cadmium (Cd) is a major anthropogenic pollutant derived from agricultural and industrial activities, including wastewater irrigations, mining and smelting of metalliferous ores.1 Due to its non-degradability, chemical mobility and high toxicity to biota, Cd can transfer through food chains and then cause various diseases to plants, animals and even human beings.2 Given that Cd contamination has posed an unprecedented threat to a wide range of ecosystems and human health, more and more attention has been globally focused on the mechanisms of Cd contamination and remediation technologies.3,4Phytoremediation, a technology of applying vegetations to remediate contaminated soils, is generally considered as a low-cost, eco-friendly approach which has gained considerable interests worldwide.5,6 Although, large amounts of plant species could hyperaccumulate heavy metals in their tissues, there still exist limitations of phytoremediation in practice such as a lower effectiveness than mechanical methods, phytotoxicity, low biomass production and limited contaminant absorption.7 Given this, the success of phytoremediation of heavy metals depends not only upon the potential of the plants' tolerance to high concentrations of heavy metals, but also upon a large plant biomass.8 In fact, the accumulation effect and tolerance of the plant still need to be strengthened in the actual repair applications, and adding exogenous substances gradually became the focus of the phytoremediation in recent years. Several chemical amendments, including ethylene diamine tetracetic acid (EDTA), citric acid (CA) have been used to promote either phytostabilization or phytoextraction process.9 As is known, EDTA is proved to be the most effective chelating agent, which is widely applied to remediate heavy metal contaminated soil.10–12 However, due to the low biodegradability and high solubility, EDTA leads to high environmental risk of heavy metal leaching to groundwater.13 In order to construct a clean and environmental friendly remediation in practical applications, biodegradable chelants and metal-tolerant plant-microbe have been the objective of particular attention. Therefore, selection of suitable chelants for the solubilization of heavy metals must be the first issue to be considered to increase extraction efficiency.
Recently, the focus of researches on chelant-enhanced phytoextraction has been shifted to some biodegradable chelating agent such as nitrilotriacetic acid (NTA), which has been used as detergents in the last 50 years. NTA can improve the uptake of metals by plants and limit leaching of metal into deeper soil.14 Several studies have been performed using NTA as a ligand to improve the efficiency of metal phytoextraction. As reported early, NTA performed effectively in desorbing Cu, Pb and Zn from soils, increasing Cu, Pb and Zn uptakes in shoots of Festuca arundinacea, and improving Cd accumulation and translocation in Siegesbeckia orientalis L.15–17 Nevertheless, little information is available about the addition of NTA to ramie under cadmium stress. In addition, in the remediation of contaminated soil, another promising alternative to amendments could be the utilization of microbe-mediated processes, because the microbial metabolites in the rhizosphere can facilitate plant metal uptake by altering the bioavailability and mobility.5 Growth-promoting bacteria can be exploited to facilitate phytoremediation.18,19 Besides, plant-associated bacteria can accelerate metal uptake and plant growth due to its feasibility of microorganisms for bioaccumulating metals from contaminated soils or its influences on metal mobilization/immobilization.20 In addition, compared with some chemical amendments living around the plant surface, the microbial metabolites are more biodegradable, and less toxic and the microbes may be possible to produce plant growth substances such as siderophores, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, and these substances improve the growth of the plant in metal contaminated soils.21,22 Combination of microbes and plants has been applied to the cleanup of contaminated soils.5,22 Despite a large number of literatures concerning the application of bacteria or endophytes in various plants, little information is available on the response of P. aeruginosa ATCC 9027 of ramie under Cd stress. As a consequence, researches about the effect and mechanism of the application of P. aeruginosa ATCC 9027 and NTA is urgently needed.
Boehmeria nivea (L.) Gaud (ramie) was applied as the study plant which is a Cd-tolerant species with large biomass and fast growth rate.23 Although there are some previous researches concerning the response of ramie to Cd toxicity in hydroponic condition, little information is available on the Cd accumulation and tolerance mechanism of ramie in the presence of microbe and NTA.24 The main objective of the research was (i) to investigate the potential ability of ramie in enhancing phytoremediation of Cd by application of P. aeruginosa ATCC 9027 and NTA; (ii) to explain the influence of P. aeruginosa ATCC 9027 and NTA on phytoremediation by analyzing physiological parameters and relevant enzymatic antioxidants of ramie; (iii) to compare P. aeruginosa ATCC 9027 and NTA, choosing a better way on practical application in phytoremediation of cadmium polluted soils in the future.
2. Materials and methods
2.1. Experimental design and treatments
The experimental soil was collected from the superficial layer (depth: 0–25 cm) originating from Taozi Lake, which located at Hunan University (Changsha, China). Soil samples were air-dried, ground, and sieved to <2 mm prior to use. The physicochemical properties of experimental soil are shown in Table 1. Then the soil was uniformly spiked with 30 and 50 mg Cd kg−1 (from solutions of Cd(NO3)2·4H2O) respectively. After incubated for 1 month, each 2 kg of the treated soil was filled into 3 L plastic pots. Ramie seeds were obtained from Chinese Academy of Agricultural Sciences, Hunan, China. After the ramie seedlings acclimated for 1 week, plants were inoculated with the Gram-negative strain P. aeruginosa ATCC 9027 (S1, S2, S3). S1 represented the addition of strain one time, while S2 represented twice and S3 represented three times, each added for one week apart. Simultaneously, 1 week before harvested, the pots were correspondingly treated with different concentrations of NTA (0, 5, 10, and 20 mmol kg−1 soil in a 200 mL solution) to the surface of the soil. The plants were performed in triplicates and conducted in a completely randomized design following fifteen treatments in 30 mg kg−1 Cd (Cd30) and 50 mg kg−1 Cd (Cd50) soil. The fifteen treatments were detailed presented in Table 2. All of the experiments were carried out in naturally illuminated greenhouse with 16 h light period at a minimal light intensity of 300 μmol m−2 s−1, temperature of 25 ± 3 °C and 60–70% relative humidity. The ramie seedlings were cultivated in Cd contaminated soil for 3 weeks. After harvested, the plants were separated into roots, stems and leaves, and frozen immediately in −80 °C for further analysis.| pH | Organic matter | Total N | Total P | CEC | Cd |
|---|---|---|---|---|---|
| 6.6 | 19.3 g kg−1 | 0.890 g kg−1 | 0.236 g kg−1 | 16.7 cmol kg−1 | Undetected |
| Number | Treatment |
|---|---|
| a NTA represented nitrilotriacetic acid; strain 1 represented the addition of strain one time, strain 2 represented twice and strain 3 represented three times. Each added for one week apart. | |
| 0 | Control |
| Cd30 (control) | 30 mg kg−1 Cd |
| 30N5 | 30 mg kg−1 Cd + 5 mmol kg−1 NTA |
| 30N10 | 30 mg kg−1 Cd + 10 mmol kg−1 NTA |
| 30N20 | 30 mg kg−1 Cd + 20 mmol kg−1 NTA |
| 30S1 | 30 mg kg−1 Cd + strain 1 |
| 30S2 | 30 mg kg−1 Cd + strain 2 |
| 30S3 | 30 mg kg−1 Cd + strain 3 |
| Cd50 (control) | 50 mg kg−1 Cd |
| 50N5 | 50 mg kg−1 Cd + 5 mmol kg−1 NTA |
| 50N10 | 50 mg kg−1 Cd + 10 mmol kg−1 NTA |
| 50N20 | 50 mg kg−1 Cd + 20 mmol kg−1 NTA |
| 50S1 | 50 mg kg−1 Cd + strain 1 |
| 50S2 | 50 mg kg−1 Cd + strain 2 |
| 50S3 | 50 mg kg−1 Cd + strain 3 |
2.2. Microbial culture
The Gram-negative strain P. aeruginosa ATCC 9027 which used as foreign substances in the work was procured from the American Type Culture Collection. It was maintained on 3–4 °C peptone agar slants and activated at 30 °C before used. P. aeruginosa ATCC 9027 inoculums from peptone agar slant were transferred to 50 mL mineral salt medium (MSM) with 0.5 g L−1 yeast extract in a 250 mL Erlenmeyer flask and performed at 37 °C on a gyratory shaker at 200 rpm for 24 h.25 Then 5 mL of the enriched cell suspension was further transferred to 100 mL MSM in 500 mL Erlenmeyer flask containing 20 g L−1 glucose as the sole carbon source.25 This inoculated culture medium was grown at 37 °C for 72 h under shaking conditions (200 rpm) which was composed of 5.0 g L−1 NH4Cl, 0.5 g L−1 MgSO4·7H2O, 2.5 g L−1 K2HPO4, 5.0 g L−1 Na2HPO4, with a pH of 6.8.26 The bacterial cells were collected by centrifugation (7000 rpm) at 4 °C for 15 min, washed twice with physiological water and obtained an inoculum with approximate absorbance value (OD600) of 0.6 (approximate 108 CFU−1 mL).27 5 mL of this strain was used for inoculation of each pot.2.3. Metal analysis
Upon harvesting, the samples were washed with deionized water and the roots were then rinsed with 5 mM CaCl2 for approximately 5 min to remove the metals absorbed.13 The plants were separated into roots, stems, and leaves. Then the samples were oven-dried, milled, and digested with a mixture of HNO3/HClO4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) by graphite digestion instrument (SISP, DS-360, China). The Cd concentration of each solution was determined using flame atomic absorption spectroscopy (FAAS) (AAnalyst 700, Perkin Elmer, USA). The translocation factor (TF) is defined as the total metal content in plant from root to shoot.28
1, v/v) by graphite digestion instrument (SISP, DS-360, China). The Cd concentration of each solution was determined using flame atomic absorption spectroscopy (FAAS) (AAnalyst 700, Perkin Elmer, USA). The translocation factor (TF) is defined as the total metal content in plant from root to shoot.28
2.4. Determination of chlorophyll and malondialdehyde (MDA) content
The chlorophyll content of ramie leaf was determined using the acetone method.29 Frozen leaf tissues were homogenized in 80% ice-cold acetone in dark and then centrifuged at 2000 rpm for 10 min. Then, chlorophyll content was determined spectro-photometrically on the supernatant at wavelength of 646 nm and 663 nm.29The MDA content of leaves was determined using the thiobarbituric acid (TBA) method.30 Frozen leaf tissues (0.5 g) were homogenized with 10 mL 10% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Then 2 mL of the aliquot of the supernatant and 2 mL of 10% TCA containing 0.5% (w/v) TBA were added. The mixtures were incubated at 95 °C for 30 min and then cooled quickly in an ice-bath. The samples were centrifuged at 10
000 rpm for 10 min. Then 2 mL of the aliquot of the supernatant and 2 mL of 10% TCA containing 0.5% (w/v) TBA were added. The mixtures were incubated at 95 °C for 30 min and then cooled quickly in an ice-bath. The samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and the absorbance of the supernatant was measured at 532 nm and corrected for nonspecific absorbance at 600 nm. The concentration of MDA was calculated using 155 mM−1 cm−1 as extinction coefficient.
000 rpm for 15 min and the absorbance of the supernatant was measured at 532 nm and corrected for nonspecific absorbance at 600 nm. The concentration of MDA was calculated using 155 mM−1 cm−1 as extinction coefficient.
2.5. Soil enzyme activities
Urease activity was determined according to the method suggested by Tabatabai and Bremner (1972), using 5.00 g of soil (DW).31 Triplicate samples of air-dried soil were measured to mix with 1 mL toluene for 15 min. Afterwards they were added to 10 mL of 10% urea and 20 mL citrate buffer (pH 6.7), and then set in the incubator at 38 °C for 24 h. After the incubation, the mixtures were immediately filtered. The filtrate was measured to determine urease activity. The absorbance was analyzed in the supernatant at 578 nm and expressed as NH4+–N (mg kg−1 h−1).322.6. Enzymatic analyses
The activity of antioxidant enzyme superoxide dismutase (SOD) and catalase (CAT) was determined with an assay kit purchased from Nanjing Jian Cheng Bioengineering Institute, Nanjing, China.Fresh leaves (0.2 g) were homogenized in 4 mL ice-cold 50 mM phosphate buffer (pH 7.0–7.4). After the centrifugation at 3500–4000 rpm at 4 °C for 10 min, the supernatant of homogenate was measured to determine SOD assays. For CAT assays, fresh leaves (0.2 g) were homogenized in 1.8 mL ice-cold normal saline (NS). After the centrifugation at 2500 rpm at 4 °C for 10 min, the supernatant was taken for detection. Total soluble protein content was determined by following the method of Bradford (1976), using bovine serum as standard.33
2.7. Statistical analysis
The data were performed by using standard statistical software (SPSS 12.0), and values were presented as the mean values ± SD of three replications. Evaluation of significant differences among different treatments was analyzed using one-way ANOVA followed by Duncan's multiple-range test, with p < 0.05 indicating statistical significance.3. Results and discussion
3.1. Plant growth and biomass
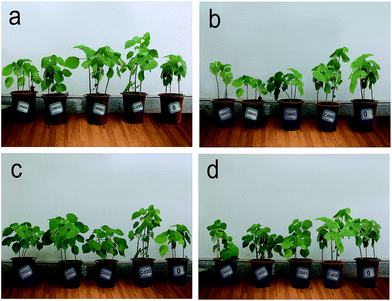
After grown for 3 weeks, the average height of plants was 51 cm, and total dry weight was 4.4 g per pot, and the average root weight accounted for 38.3% of the total biomass. As Fig. 1 exhibited, the growth state of ramie showed differences among the different treatments. The addition of NTA to the soil inhibited the plant biomass when plants were exposed to Cd30 and Cd50, but no visible toxic symptoms appeared except the treatment of 50N20. In the 50N20 group, whitish-brown chlorosis and necrosis appeared. While the addition of strain was found to significantly enhance plant growth. Not only was there a positive biomass production, but also there was a higher and verdant growth. Moreover, the biomass of ramie treating with strain even exceeded the biomass production under unpolluted soil treatment.As seen from Fig. 2, significant differences in the biomass of shoot and root were observed among the 15 treatments (p < 0.05). The biomass of ramie was decreased after the addition of different levels of NTA in Cd30 and Cd50 treatments, but there was an exception in the group of 30N5, which had an increased biomass of 10% compared to the control plants. However, when strain was added to the soil, the total dry biomass was increased ranging from 15.8% to 33.1% and 14.3% to 30.7% under the level of Cd30 and Cd50 respectively. These results demonstrated that high concentration of NTA inhibited ramie biomass, while the application of P. aeruginosa ATCC 9027 could enhance the total dry biomass in presence of Cd contamination.
 | ||
| Fig. 2 The differences in leaf, stem and root biomass of 15 treatments after 3 weeks of growth in Cd contaminated soil (30 and 50 mg kg−1 Cd). All the values are mean of triplicates ± SD. | ||
The increased biomass of ramie with the application of P. aeruginosa ATCC 9027 may be beneficial for the removal of cadmium, because the more biomass means it can pick up more contaminants. The possible mechanisms involved in ramie growth promotion by P. aeruginosa ATCC 9027 could be explained in two different ways: firstly, the indirect promotion of ramie growth occurred when P. aeruginosa ATCC 9027 prevented or decreased some of the deleterious effects of phytopathogenic organism.5 Besides, P. aeruginosa ATCC 9027 can also directly promote plant growth by providing with a compound that is synthesized by the bacterium or by further facilitating the uptake of nutrients (especially small molecules such as sugars, amino acids, organic acids) from the plant.5,34 The inhibition biomass under 10 and 20 mmol kg−1 NTA was that metal phytotoxicity did occur due to the desorption and dissolution effects by NTA.35 Analogously, negative effects of NTA on plan growth were reported in many studies.36,37
3.2. Effect of Cd on physiological characteristics
The previous studies suggested that the inhibition of malondialdehyde (MDA) or the degradation of chlorophyll was responsible for the growth restraint induced by Cd.38 In addition, Cd uptake by plants has been reported to induce extensive lipid peroxidation, which reflected the degree of cell membrane damage caused by oxygen free radicals.39Not surprisingly, it can be clearly seen in Fig. 3, the MDA content was 34.3 nmol g−1 FW in unpolluted soil treatment, but reached up to 63.7 nmol g−1 FW and 74.1 nmol g−1 FW in Cd30 and Cd50 treatments, respectively. The NTA treatments exhibited a linear enhancement of MDA content which was in accordance with the increase in concentration of NTA. There was a slight decline in MDA content at low NTA concentrations (5 mmol kg−1) but higher MDA content was detected in ramie when treated with 10 and 20 mmol kg−1 NTA compared to the controls. The increase of MDA content is probably due to its poisonous derivatives and the deleterious effect of H2O2.40 In addition, it can be seen that MDA contents in the leaves of ramie with different levels of strain were lower than the controls, although differences were not statistically significant. Similarly, Serratia nematodiphila LRE07, a endophytic bacteria, significantly attenuated the content of MDA in Solanum nigrum L.41 The lower level of MDA in leaves with the application of strain revealed that bacterial inoculation can alleviate the damage on the cell membrane caused by Cd stress.
The chlorophyll content in plants was determined to elucidate the toxic effect of Cd or exogenous chelants on photosynthesis system in ramie (Fig. 3b). Chlorophyll content in leaves of ramie showed no significant alteration (p > 0.05) when added with NTA under Cd30, but decreased when ramie was exposed to Cd50. In contrast, both in Cd30 and Cd50 treatments, there were a slight increase in chlorophyll content with the addition of P. aeruginosa ATCC 9027. These results meant that ramie suffered strong stress with NTA while alleviated with P. aeruginosa ATCC 9027. These are in consistent with some previous studies, which have also reported that bacterial strain could positively influence the chlorophyll contents of host plant under abiotic stresses.34,42
3.3. Cd accumulation and distribution in ramie
Fig. 4 presented the effects of 15 different treatments on accumulation of cadmium in different tissues of ramie. The P. aeruginosa ATCC 9027 or NTA improved the accumulation of Cd in shoots and roots of ramie to the different degrees. Generally speaking, in different tissues of ramie, Cd accumulations in roots were considerable higher than in shoots, which was in agreement with previous reports on ramie.43 The performance of NTA did not display obvious effect in Cd uptake. Although increasing concentrations of NTA under Cd30 and Cd50 led to an increase of the total Cd accumulations in plants, there was an inhibition of Cd accumulation in the roots of ramie when compared with Cd30 and Cd50 treatments (except for the treatment of 30N20). But the accumulation of Cd in shoot with NTA treatments was significantly improved compared with Cd50 treatments. The highest Cd accumulation in shoot with NTA was 274.5 and 405.1 mg kg−1 DW in 30N20 and 50N20 treatments, respectively. From Fig. 4, the infected strain plants in the presence of Cd had higher Cd concentration in tissues compared with non-inoculation controls, especially in the roots. And the higher concentration of Cd was observed in plants under Cd50 than that under Cd30 with the addition of strain. The highest Cd accumulation in ramie with P. aeruginosa ACCT 9027 was 748.3 and 1112.6 mg kg−1 DW in 30S3 and 50S2 treatments, respectively. Besides, the inoculation of P. aeruginosa ATCC 9027 increased accumulation in root ranging from 54% to 96% and 13% to 104% in 30 and 50 mg kg−1 Cd soils, respectively. The average uptake of Cd in root with P. aeruginosa ACCT 9027 was increased by approximately 1.54- to 1.96-fold and 1.13- and 2.04-fold under Cd30 and Cd50 treatments, respectively. Furthermore, the average accumulation of Cd with P. aeruginosa ATCC 9027 was about 1.95-fold (Cd30) and 1.54-fold (Cd50) compared to the corresponding NTA treatments (Table 3). | ||
| Fig. 4 Changes in Cd amounts in the leaf, stem and root of ramie under different treatments. All the values are mean of triplicates ± SD. | ||
| Treatments | Cd content (mg kg−1 DW) | TF value | ||
|---|---|---|---|---|
| Leaves | Stems | Roots | ||
| 0 | UD | UD | UD | — |
| Cd30 | 242.93 ± 7.92 | 136.87 ± 7.78 | 29.76 ± 2.12 | 0.272 |
| 30N5 | 43.85 ± 2.33 | 69.50 ± 2.12 | 107.85 ± 5.87 | 0.420 |
| 30N10 | 35.62 ± 1.70 | 108.46 ± 2.68 | 167.38 ± 3.11 | 0.345 |
| 30N20 | 67.52 ± 1.96 | 206.57 ± 4.94 | 267.53 ± 7.78 | 0.41 |
| 30S1 | 29.45 ± 1.91 | 255 ± 4.24 | 393.5 ± 6.36 | 0.292 |
| 30S2 | 26.85 ± 0.64 | 272 ± 15.55 | 373.5 ± 8.49 | 0.321 |
| 30S3 | 35.25 ± 2.47 | 236.25 ± 13.08 | 476.75 ± 6.01 | 0.228 |
| Cd50 | 55.12 ± 1.57 | 185.75 ± 4.74 | 333.95 ± 7.85 | 0.288 |
| 50N5 | 95.01 ± 6.47 | 190.75 ± 7.42 | 195.31 ± 8.49 | 0.586 |
| 50N10 | 85.95 ± 2.19 | 311.52 ± 19.79 | 257.75 ± 5.30 | 0.615 |
| 50N20 | 128.25 ± 4.59 | 276.75 ± 6.72 | 267.35 ± 5.44 | 0.451 |
| 50S1 | 17.905 ± 0.74 | 358.25 ± 6.01 | 613.5 ± 6.36 | 0.245 |
| 50S2 | 25.355 ± 0.69 | 405.5 ± 13.43 | 680.75 ± 18.74 | 0.254 |
| 50S3 | 28.69 ± 0.08 | 279.5 ± 9.192 | 378 ± 9.89 | 0.327 |
The TF of the heavy metal Cd and the applied chelating agents NTA and P. aeruginosa ATCC 9027 are depicted in Table 2. Compared to the Cd30 and Cd50 treatments, the addition of NTA tended to significantly increase Cd concentration in stems and leaves, indicating that NTA enhanced Cd translocation from roots to shoots. More interestingly, the TF of 50N (50 mg kg−1 Cd and NTA) was higher than 30N (30 mg kg−1 Cd and NTA). NTA increased TF compared to the controls ranked 17.4–36.2%, 74.5–81.8% at Cd30 and Cd50, respectively. The increase of TF might be attributed to the fact that NTA facilitated Cd movement from roots to shoots. This is the greatest advantage of NTA compared to other chelating agent for the remediation of contaminated soils. Because ramie obviously absorbed Cd in root, so the application of P. aeruginosa ATCC 9027 to soil caused no obvious difference of TF.
The present investigation confirmed that strain is a better effective chelator than NTA in accumulating Cd as well as increasing its availability for plant uptake. The ability of NTA to desorb metals from the soil was lower in comparison to strain due to the low affinity constants of its complexes with Cd.15 This is consistent with previous research which have also reported that bacteria inoculation could enhance plant to absorb heavy metals.44,45 Overall the microbial activities in the root soils enhance the efficiency of phytoremediation mechanisms under Cd stress soil by two complementary ways: (i) plant associated microbes reduce the mobility or availability of pollutants in the rhizosphere; (ii) the microbes confer plant metal tolerance and/or increase the plant biomass production in order to remove the pollutants.5,21 This can be interpreted as that the treatments with P. aeruginosa ATCC 9027 in ramie can produce iron chelators called siderophores in response to low iron levels in plants.5 Plant growth-promoting bacteria may synthesize siderophores which can sequester and solubilize iron from the soils and provide it to plant cells.21,34 However, further investigations on how the plant-associated metabolites producing microbes influence the heavy metal mobilization and it uptake by plants in contaminated soils are needed. These processes were therefore reasoned that the strain had an exceptional capacity to accumulate Cd in the developed root system in plants. In addition, NTA acted as a chelating agent which was useful to facilitate Cd movement. And the results are in agreement with some previous studies which had also reported that the addition of NTA could promote the mobilization of heavy metals.46,47 The increased TF by NTA was probably due to the following reasons. Firstly, plants accumulate free metals in their roots in the time period before chelant application. Secondly, with the application of a chelating agent, metals are complexed within the roots and translocated as metal chelates.48
3.4. Soil enzyme activities
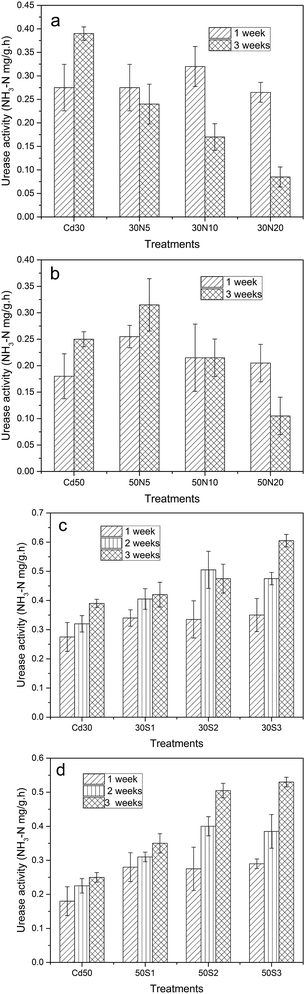
Many previous studies correlated with the toxicity of heavy metals on enzyme activities especially urease activity in soil are available in literatures.49–51 Additionally, enzyme activities have been suggested as sensitive indicators of soil quality, which indicated that urease activity can be considered as the biochemical index which reflects the degree of soil Cd pollution.52Fig. 5 illustrated the urease activity changes in the soil during the plant growth. When NTA was added to the soil, the urease activity significantly decreased with increasing culture time (Fig. 5a and b). It suggested that the urease activity tended to be decreased with increasing concentration of NTA when exposed to Cd30, and the lowest urease activity of ramie appeared in 30N20 treatment after 3 weeks. When treated with Cd50, the low dose of NTA (5 mmol kg−1) enhanced urease activity, while the high dose of NTA (20 mmol kg−1) decreased urease activity. The results suggested that urease activity was lower at high concentration of NTA than in other treatments. Besides, the higher the concentration of NTA is, the more obvious effect can be seen. Fig. 5c and d showed that the urease activity was maximized after 3 weeks (except for 30S2) when exposed to Cd30 and Cd50. Moreover, there was a net increase in urease activity from 1 week to 3 weeks with the increase of strain concentration. It means that strain-infection positively influenced the urease activity of soil. Furthermore, there was a negatively correlation between the Cd content and the urease activity which was also confirmed by Stpniewska et al.53 The increase of urease activity in soil with the application of strain could be explained that bacteria can produce a variety of low molecular weight organic acids such as chelate compounds or complexes, and consequent release of active urease molecules.54
3.5. Effect of Cd on activated oxygen metabolism
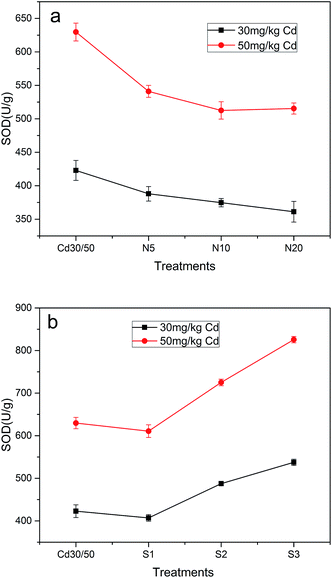
Antioxidant enzymes (SOD, CAT) removing the cells from active oxygen species were determined as pivotal enzymes (Fig. 6). SOD was considered as first defense barrier against reactive oxygen species (ROS) as it acted on superoxide radicals.55 It is essential to control the levels of ROS for their cellular damage activities. Fig. 6a showed that SOD activity in leaves of ramie which was treated with NTA was reduced, and the maximum decrease rate in the treatment of 30N20 and 50N10 were up to 14.6% and 18.6%, respectively. When strain was inoculated, SOD activity was increased with increasing concentration of strain, and the maximum increase rate was up to 26.3% in the 30S3 treatment and 37.1% in the 50S3 treatment compared to Cd treatments, respectively. Fig. 7 represents CAT activity in leaves of ramie which treated with NTA and strain. It can be seen that strain induced obvious decrease in CAT activity. In the present study, when ramie seedlings were submitted to different levels of NTA (5, 10, 20 mmol kg−1), obvious decrease in the activities of CAT was observed with the addition of NTA by 5 and 10 mmol kg−1 while an increase was observed at NTA concentration of 20 mmol kg−1. Fig. 7b showed that CAT activity was lower under strain treatment than that under single Cd treatment. The increase of CAT activity was probably due to the fact that heavy metals stimulated the synthesis of enzyme. | ||
| Fig. 7 Changes of catalase activity (CAT) in leaves of ramie added with NTA (a), and infected by P. aeruginosa ATCC 9027 (b) exposed to 30, 50 mg kg−1 Cd. All the values are mean of triplicates ± SD. | ||
The results indicated that NTA and antioxidative enzymes activities were negatively correlated, while ATCC 9027 could promote ramie against Cd phytotoxicity via improving antioxidative enzymes activities. Similar effects of bacteria on plant antioxidative system have also been reported for Cd in drunken horse grass and Zn in ryegrass.56 This could be explained that SOD dismutates two superoxide radicals to oxygen and H2O2 and thus maintained superoxide radicals in steady state level.56 The reason for decrease of SOD activity with NTA might be the inactivation of enzyme by H2O2.40 The increase of SOD activity with strain was attributed to the synthesis of enzyme protein.57 Moreover, some traits, such as production of siderophore and antioxidative enzymes, of bacteria may be the possible reason of enhancing the activities of antioxidative enzymes in plants.58 CAT exists in mitochondria and peroxisomes where it decomposes H2O2 to water and oxygen. The increasing in CAT activity was probably due to the fact that heavy metals stimulated the synthesis of enzyme, the decline of CAT activity might be attributed to inactivation of enzyme by ROS.59 These results indicated that the inoculation with beneficial microbes assisted plants to alleviate heavy metal stress through enhancing the activities of antioxidant enzymes.
4. Conclusions
The results demonstrated that the addition of NTA effectively increased the Cd translation from root to shoot, whereas showed no obvious effect in Cd uptake, even plant growth and related enzyme activities were inhibited. However, the inoculation of P. aeruginosa ATCC 9027 alleviated these Cd-induced damages, resulting in promotion of ramie growth, improvement of antioxidative enzymes activities and increase of total Cd-uptake by ramie. Additionally, the average accumulation of Cd by ramie with P. aeruginosa ATCC 9027 treatment was much higher than that of NTA treatments. The improvement in antioxidative enzymes activities and urease activity in soil after the inoculation of P. aeruginosa ATCC 9027 are probably the main mechanisms involved in Cd phytotoxicity reduction. Besides, the results showed that the inoculation with beneficial microbes assisted plants to alleviate heavy metal stress through enhancing the activities of antioxidant enzymes. All these results indicated that P. aeruginosa ATCC 9027 may be an effective remedy for Cd contaminated soils and a promising candidate for practical application on phytoremediation of Cd contaminated soils. However, additional studies regarding the interaction of plant–bacterial–metals in polluted soils are needed in the further investigations.Acknowledgements
The authors would like to thank financial support from the National Natural Science Foundation of China (Grant No. 41271332 and 51478470), and the Fundamental Research Funds for the Central University, Hunan University.References
- S. McGrath, F. Zhao and E. Lombi, Plant Soil, 2001, 232, 207–214 CrossRef CAS.
- G. de la Rosa, J. R. Peralta-Videa, M. Montes, J. G. Parsons, I. Cano-Aguilera and J. L. Gardea-Torresdey, Chemosphere, 2004, 55, 1159–1168 CrossRef CAS PubMed.
- S. Satarug, J. R. Baker, P. E. Reilly, M. R. Moore and D. J. Williams, Arch. Environ. Health, 2002, 57, 69–77 CrossRef CAS PubMed.
- S. S. Gill and N. Tuteja, Plant Signaling Behav., 2011, 6, 215–222 CrossRef CAS.
- B. R. Glick, Biotechnol. Adv., 2010, 28, 367–374 CrossRef CAS PubMed.
- E. L. Arthur, P. J. Rice, P. J. Rice, T. A. Anderson, S. M. Baladi, K. L. Henderson and J. R. Coats, Crit. Rev. Plant Sci., 2005, 24, 109–122 CrossRef CAS PubMed.
- S. L. Doty, New Phytol., 2008, 179, 318–333 CrossRef CAS PubMed.
- H. Grčman, Š. Velikonja-Bolta, D. Vodnik, B. Kos and D. Leštan, Plant Soil, 2001, 235, 105–114 CrossRef.
- O. Barrutia, C. Garbisu, J. Hernández-Allica, J. I. García-Plazaola and J. M. Becerril, Environ. Pollut., 2010, 158, 1710–1715 CrossRef CAS PubMed.
- S. Doncheva, M. Moustakas, K. Ananieva, M. Chavdarova, E. Gesheva, R. Vassilevska and P. Mateev, Environ. Sci. Pollut. Res., 2013, 20, 823–833 CrossRef CAS PubMed.
- H. Zaier, T. Ghnaya, K. B. Rejeb, A. Lakhdar, S. Rejeb and F. Jemal, Bioresour. Technol., 2010, 101, 3978–3983 CrossRef CAS PubMed.
- M. M. Meighan, T. Fenus, E. Karey and J. MacNeil, Chemosphere, 2011, 83, 1539–1545 CrossRef CAS PubMed.
- Z. Zhao, M. Xi, G. Jiang, X. Liu, Z. Bai and Y. Huang, J. Hazard. Mater., 2010, 181, 455–459 CrossRef CAS PubMed.
- D. Leštan, C.-l. Luo and X.-d. Li, Environ. Pollut., 2008, 153, 3–13 CrossRef PubMed.
- M. F. Quartacci, B. Irtelli, A. J. Baker and F. Navari-Izzo, Chemosphere, 2007, 68, 1920–1928 CrossRef CAS PubMed.
- S. Zhao, F. Lian and L. Duo, Bioresour. Technol., 2011, 102, 621–626 CrossRef CAS PubMed.
- J. Lan, S. Zhang, H. Lin, T. Li, X. Xu, Y. Li, Y. Jia and G. Gong, Chemosphere, 2013, 91, 1362–1367 CrossRef CAS PubMed.
- L. A. Newman and C. M. Reynolds, Trends Biotechnol., 2005, 23, 6–8 CrossRef CAS PubMed.
- L. Chen, S. Luo, X. Xiao, H. Guo, J. Chen, Y. Wan, B. Li, T. Xu, Q. Xi and C. Rao, Appl. Soil. Ecol., 2010, 46, 383–389 CrossRef PubMed.
- Y. Gao, P. Zhou, L. Mao, W. Shi and Y. Zhi, Russ. J. Plant Physiol., 2010, 57, 501–508 CrossRef CAS.
- M. Rajkumar, S. Sandhya, M. N. Prasad and H. Freitas, Biotechnol. Adv., 2012, 30, 1562–1574 CrossRef CAS PubMed.
- Z.-l. He and X.-e. Yang, J. Zhejiang Univ., Sci., B, 2007, 8, 192–207 CrossRef PubMed.
- X. Wang, Y. Liu, G. Zeng, L. Chai, X. Song, Z. Min and X. Xiao, Environ. Exp. Bot., 2008, 62, 389–395 CrossRef CAS PubMed.
- M. Wójcik, J. Vangronsveld and A. Tukiendorf, Environ. Exp. Bot., 2005, 53, 151–161 Search PubMed.
- H. Zhong, Y. Jiang, G. Zeng, Z. Liu, L. Liu, Y. Liu, X. Yang, M. Lai and Y. He, J. Hazard. Mater., 2015, 285, 383–388 CrossRef CAS PubMed.
- I. Siegmund and F. Wagner, Biotechnol. Tech., 1991, 5, 265–268 CrossRef CAS.
- Y. Wan, S. Luo, J. Chen, X. Xiao, L. Chen, G. Zeng, C. Liu and Y. He, Chemosphere, 2012, 89, 743–750 CrossRef CAS PubMed.
- S. P. McGrath and F.-J. Zhao, Curr. Opin. Biotechnol., 2003, 14, 277–282 CrossRef CAS.
- H. K. Lichtenthaler, Methods Enzymol., 1987, 350–382 CAS.
- A. Chaoui, S. Mazhoudi, M. H. Ghorbal and E. El Ferjani, Plant Science, 1997, 127, 139–147 CrossRef CAS.
- M. A. Tabatabai and J. M. Bremner, Soil Biol. Biochem., 1972, 4, 479–487 CrossRef CAS.
- E. Kandeler and H. Gerber, Biol. Fertil. Soils, 2007, 69, 99–107 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- S. Ghosh, J. N. Penterman, R. D. Little, R. Chavez and B. R. Glick, Plant Physiol. Biochem., 2003, 41, 277–281 CrossRef CAS.
- M. F. Quartacci, A. J. M. Baker and F. Navari-Izzo, Chemosphere, 2005, 59, 1249–1255 CrossRef CAS PubMed.
- B. H. Robinson, T. M. Mills, D. Petit, L. E. Fung, S. R. Green and B. E. Clothier, Plant Soil, 2000, 227, 301–306 CrossRef CAS.
- E. Meers, M. Hopgood, E. Lesage, P. Vervaeke, F. M. G. Tack and M. G. Verloo, Int. J. Phytorem., 2004, 6, 95–109 CrossRef CAS PubMed.
- Y. Liu, X. Wang, G. Zeng, D. Qu, J. Gu, M. Zhou and L. Chai, Chemosphere, 2007, 69, 99–107 CrossRef CAS PubMed.
- B. Somashekaraiah, K. Padmaja and A. Prasad, Physiol. Plant., 1992, 85, 85–89 CrossRef CAS PubMed.
- L. Sandalio, H. Dalurzo, M. Gomez, M. Romero-Puertas and L. Del Rio, J. Exp. Bot., 2001, 52, 2115–2126 CAS.
- J. Dong, F. Wu and G. Zhang, Chemosphere, 2006, 64, 1659–1666 CrossRef CAS PubMed.
- X. Zhang, C. Li and Z. Nan, J. Hazard. Mater., 2010, 175, 703–709 CrossRef CAS PubMed.
- Z. Sun, Y. Liu, Y. Huang, G. Zeng, Y. Wang, X. Hu and L. Zhou, Journal of Ecological Engineering, 2014, 71, 108–112 CrossRef PubMed.
- H. B. Bradl, J. Colloid Interface Sci., 2004, 277, 1–18 CrossRef CAS PubMed.
- M. Malandrino, O. Abollino, A. Giacomino, M. Aceto and E. Mentasti, J. Colloid Interface Sci., 2006, 299, 537–546 CrossRef CAS PubMed.
- K. Chiu, Z. Ye and M. Wong, Chemosphere, 2005, 60, 1365–1375 CrossRef CAS PubMed.
- M. F. Quartacci, A. J. Baker and F. Navari-Izzo, Chemosphere, 2005, 59, 1249–1255 CrossRef CAS PubMed.
- B. D. Ensley, M. J. Blaylock, S. Dushenkov, N. P. B. A. Kumar and Y. Kapulnik, US Pat., 5917117, 1999.
- F. Kandeler, C. Kampichler and O. Horak, Biol. Fertil. Soils, 1996, 23, 299–306 CrossRef.
- T. Stuczynski, G. McCarty and G. Siebielec, J. Environ. Qual., 2003, 32, 1346–1355 CrossRef CAS.
- S. Chaperon and S. Sauve, Soil Biol. Biochem., 2007, 39, 2329–2338 CrossRef CAS PubMed.
- A. Guwy, S. Martin, F. Hawkes and D. Hawkes, Enzyme Microb. Technol., 1999, 25, 669–676 CrossRef CAS.
- Z. Stpniewska, A. Wolińska and J. Ziomek, J. Environ. Sci., 2009, 21, 1142–1147 CrossRef.
- F. Cattaneo, P. Di Gennaro, L. Barbanti, C. Giovannini, M. Labra, B. Moreno, E. Benitez and C. Marzadori, Appl. Soil. Ecol., 2014, 84, 213–222 CrossRef PubMed.
- R. G. Alscher, N. Erturk and L. S. Heath, J. Exp. Bot., 2002, 53, 1331–1341 CrossRef CAS.
- M. Bonnet, O. Camares and P. Veisseire, J. Exp. Bot., 2000, 51, 945–953 CrossRef CAS.
- S. Verma and R. Dubey, Plant Sciences, 2003, 164, 645–655 CrossRef CAS.
- K. Shah, R. G. Kumar, S. Verma and R. Dubey, Plant Sciences, 2001, 161, 1135–1144 CrossRef CAS.
- A. M. Reddy, S. G. Kumar, G. Jyothsnakumari, S. Thimmanaik and C. Sudhakar, Chemosphere, 2005, 60, 97–104 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |