Silver and gold nanoparticles from Sargentodoxa cuneata: synthesis, characterization and antileishmanial activity
Abstract
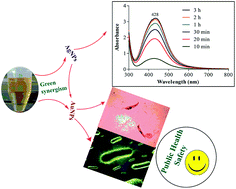
Leishmaniasis remains one of the fatal diseases worldwide and the conventional antileishmanial therapies are associated with several drawbacks. Therefore, there is a need to develop new antileishmanial strategies. Biogenic silver and gold nanoparticles possess broad-spectrum antimicrobial activities and could be future alternatives to current antimicrobial agents. In this report, we present a simple and green approach to synthesize silver and gold nanoparticles with efficient biological activities. Phytochemicals from Sargentodoxa cuneata were used to reduce and stabilize the silver and gold ions into metallic nanoparticles. The synthesized nanoparticles were characterized by UV-visible spectroscopy (surface plasmon resonance), X-ray diffraction analysis (crystallinity), high-resolution transmission electron microscopy (size and morphology), energy dispersive X-ray (elemental composition) and FTIR (surface functionalities). Under optimized conditions, the synthesized silver nanoparticles were spherical in shape, of small sizes (3–8 nm) and well dispersed. However, the gold nanoparticles were mostly hexagonal in shape with approximate sizes from 15 to 30 nm. Promising antileishmanial activity was shown by silver and gold nanoparticles with an IC50 value of 4.37 and 5.29 μg mL−1 respectively. Silver nanoparticles also exhibited significant antibacterial activity against Staphylococcus aureus (32 ± 3 mm), Pseudomonas araginosis (16 ± 2 mm), and Bacillus subtilis (18 ± 2 mm). The depicted biological activities of nanoparticles are not simply due to the capped silver and gold atoms but also to their surface macromolecules. Thus, the use of Sargentodoxa cuneata as a reducing and capping agent will retain its biological activity even after the depletion of maintained silver and gold. The findings of this study indicate that these nanoparticles could be an alternative, safe, and effective source of antileishmanial agents.

 Please wait while we load your content...
Please wait while we load your content...