Enantio- & chemo-selective preparation of enantiomerically enriched aliphatic nitro alcohols using Candida parapsilosis ATCC 7330
Abstract
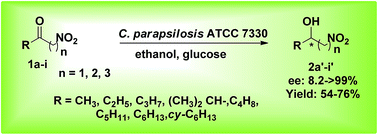
Enantiomerically pure β- and γ-nitro alcohols were prepared from their respective nitro ketones by asymmetric reduction mediated by the biocatalyst, Candida parapsilosis ATCC 7330 under optimized reaction conditions (ee up to >99%; yields up to 76%). This biocatalyst exhibits high chemoselectivity and reduces the keto group in preference to nitro group along with good enantioselectivity to produce enantiomerically enriched nitro alkanols. The asymmetric reduction of aliphatic nitro ketones was carried out in water with ethanol as cosolvent and glucose as cosubstrate using the whole cells of Candida parapsilosis ATCC 7330 in much lesser time (4 h). For the first time, the biocatalytic asymmetric reduction of the following ketones is reported here: 1-nitro-butan-2-one, 1-nitro-pentan-2-one, 3-methyl-1-nitro-butan-2-one and 1-cyclohexyl-2-nitroethanone to produce (R)-alcohols [ee up to 79%, yield up to 74%] and 1-nitro-hexan-2-one and 1-nitro-heptan-2-one to produce (S)-alcohols [ee up to 81%, yield up to 76%].

 Please wait while we load your content...
Please wait while we load your content...