Experimental and theoretical studies of DMH as a complexing agent for a cyanide-free gold electroplating electrolyte†
Abstract
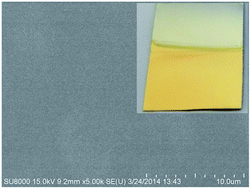
In this study, a cyanide-free gold electroplating electrolyte using 5,5-dimethylhydantoin (DMH) as a complexing agent was introduced. A golden bright gold electrodeposit with smooth and compact surface was obtained from the introduced cyanide-free gold electroplating electrolyte. The results of scanning electron microscopy (SEM) measurements confirmed that the golden bright gold electrodeposit possesses an excellent leveling capability as well as smooth and compact morphology. The crystalline structure of the gold electrodeposits was characterized by X-ray diffraction (XRD) analysis. Computational chemistry was employed to provide an insight view of the reason for selecting DMH among the various hydantoin derivatives as the complexing agent for the introduced cyanide-free gold electroplating electrolyte. Quantum chemical calculations were employed to study the electronic properties and orbital information of the investigated complexing agents. The adsorption interactions between these complexing agents and the metal surfaces were investigated by molecular dynamic (MD) simulations. Consequently, the results of these theoretical studies revealed that DMH was selected among the various hydantoin derivatives as the complexing agent for the introduced cyanide-free gold electroplating electrolyte due to its strong electron donating abilities and high adsorption energies on the metal surfaces.

 Please wait while we load your content...
Please wait while we load your content...