Mo doped porous Ni–Cu alloy as cathode for hydrogen evolution reaction in alkaline solution
Abstract
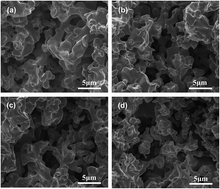
Molybdenum doped porous Ni–Cu cathodes are conveniently prepared by powder metallurgy in this work. The microporous structure of the porous Ni–Cu based cathodes has a certain correlation with the content of molybdenum, which consequently influences the electrocatalytic behavior as electrode materials. Electrocatalytic performance of the prepared cathodes has been investigated by hydrogen evolution reaction in 6.0 mol L−1 KOH solution. It is demonstrated that the Mo doped porous Ni–Cu cathodes exhibit a superior electrocatalytic activity for HER over a binary porous Ni–Cu cathode, among which the porous Ni–Cu with 6.0 wt% Mo shows the highest intrinsic activity and an ascendant stability during HER. Although the porous Ni–Cu (with 9.0 wt% Mo) expresses the highest activity, a gradual decline of the current density for HER in long-term operation is found, which is due to the dissolution of Mo from the porous skeleton in alkaline solution.

 Please wait while we load your content...
Please wait while we load your content...