A hybrid composite of gold and graphene oxide as a PCR enhancer†
Abstract
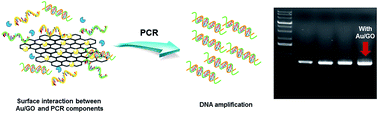
A hybrid composite of gold-decorated graphene oxide (GO) was synthesized and applied as a PCR enhancer. The analysis of the results of PCR demonstrates that the gold and graphene oxide (Au/GO) hybrid composite improves PCR performance. The chemical interaction of the hybrid composite and the PCR components and the potential mechanism behind the hybrid composite-assisted PCR were also investigated and discussed.

 Please wait while we load your content...
Please wait while we load your content...