Internal and external factors affecting the stability of glycerol monostearate structured emulsions
Fan C. Wang and
Alejandro G. Marangoni*
Department of Food Science, University of Guelph, 50 Stone Road W, N1G 2W1, Guelph, ON, Canada. E-mail: amarango@uoguelph.ca
First published on 19th October 2015
Abstract
Monoglyceride (MG) structured emulsions have been developed for use in diverse food and cosmetic applications. However, these MG-structured emulsions undergo a polymorphic transformation from the α-gel phase to the coagel phase, resulting in emulsion destabilization and water syneresis. In this study, the stability of emulsions containing 60–70% (w/w) water structured with 5% (w/w) of a blend of the emulsifier glycerol monostearate (GMS) and co-emulsifier sodium stearoyl lactylate (SSL), was assessed. The internal factors examined were concentrations of co-emulsifier and the addition of the polysaccharide xanthan gum. External factors examined included cooling rate and applied shear. The methods used to study the polymorphic transition were differential scanning calorimetry, X-ray diffraction, and pulsed proton nuclear magnetic resonance. In this work, the sub-α Coagel Index was successfully used to characterize the degree of coagel formation in MG-structured emulsions. Results showed that the stability of the α-gel phase was improved by using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w SSL
9 w/w SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS ratios and by adding 0.1% xanthan gum. Slow cooling rates without shear could also increase the stability of the α-gel phase in the structured emulsion system.
GMS ratios and by adding 0.1% xanthan gum. Slow cooling rates without shear could also increase the stability of the α-gel phase in the structured emulsion system.
1. Introduction
Monoglycerides (MGs) are lipid molecules that have one fatty acyl chain attached to a glycerol backbone, and are thus amphiphilic. They self-assemble both in water and oil into several types of mesophases, depending on temperature and concentration,1 and are used extensively in food and personal care products.1 Our group developed a MG-structured oil-in-water (o/w) emulsion that contains 3–5% (w/w) of saturated MGs, 0.15–0.25% (w/w) of co-emulsifier, 10–70% (w/w) of oil, and 20–60% (w/w) of water.2–4 This structured emulsion has been shown to function as a shortening that has no trans fat and low in saturated fat.3,4 The physical properties of these MG-structured emulsions can be tailored to replace shortening in most bakery products such as cookies,5 breads,6 and laminated puff pastries.7The structural and rheological properties of MG-structured emulsions have been studied and the lamellar phase has been found to be the optimal structure for a spreadable emulsion.4 Upon homogenization, the MGs and co-emulsifiers self-assemble into hydrated lamellar structures and form a fat-like gel network, in which oil droplets are surrounded by alternating MG-bilayers and water.3,4,8–10
However, MG-structured emulsions are prone to phase separation and water syneresis can be observed after four weeks of storage at room temperature. The emulsion destabilization is mainly caused by a polymorphic transformation of the MGs.1,11–14 In order to increase the water swelling capacity of the MG-bilayers, which could improve the stability of the MG-structured emulsion, the nature and dynamics of the polymorphic behavior of MG–water systems must be better understood.
The polymorphic behavior of glycerol monostearate (GMS) in water has been well characterized in previous work. When heating a GMS–water mixture above its Krafft temperature (Tk), GMS molecules self-assemble into an Lα liquid crystalline phase.11,13,14 Further heating of the system above 80 °C leads to the formation of cubic phases.10,14,15 Upon cooling the Lα liquid crystalline phase below Tk, the hydrocarbon chains of the GMS lose mobility and transform into an Lβ phase (α-gel phase), in which thick layers of water are retained between the MG bilayers. The α-gel phase undergoes a thermally reversible phase transition to the sub-α-gel phase when the temperature drops below 13 °C.16 However, both the sub-α-gel phase and the α-gel phase are thermodynamically unstable and will gradually crystallize into a more densely packed Lβ′ phase (the coagel phase), accompanied by a release of water from the bilayer structures.1,11,13,17
The polymorphic form of MG–water systems and MG-structured emulsions can be characterized using powder X-ray diffraction (XRD) and differential scanning calorimetry (DSC). The α-gel phase has a single peak at ∼4.2 Å in the wide angle scattering (WAXS) region and a peak representing the (001) plane at ∼55 Å in the small angle scattering region (SAXS); on the other hand, the coagel phase has several diffraction peaks between 3.6 and 4.6 Å in the WAXS region and a peak representing the (001) plane at 49 Å in the SAXS region.1 The extent of polymorphic transformation from the α-gel phase to the coagel phase can be described using the Coagel Index (CI) determined by DSC.17 In the calculation of CI, two heating cycles are applied to the MG-structured systems above Tk. The enthalpy of melting from the first heating cycle (ΔH1) represents the melting of both the α-gel and the coagel phase in aged samples while the enthalpy of melting obtained from the second heating cycle (ΔH2) represents the melting of only the freshly formed α-gel phase. The CI can then be calculated by taking the ratio of ΔH1/ΔH2.17 CI equals to 1.0 or 2.0 means that the MG-structured system is either completely in the α-gel phase or the coagel phase.17 Interestingly, CI has not been previously used to characterize the polymorphic transformation of MG in o/w structured emulsions. Another parameter, the sub-α Coagel Index (CIsubα), has been recently developed to determine the degree of coagel formation and could possibly be used to characterize the polymorphic state of MG-structured emulsions.18 Similar to the determination of CI, CIsubα is determined also from two DSC heating cycles, however, the starting temperature in these cycles are set at lower than 13 °C to ensure that the system is in the sub-α-gel phase. The enthalpies of the phase transition from the sub-α-gel phase to the α-gel phase are obtained from the first heating cycle (ΔHsubα1) and the second heating cycle (ΔHsubα2), respectively. CIsubα is then calculated from taking the ratio of ΔHsubα1/ΔHsubα2.18 CIsubα equals to 1.0 indicates that the system is completely in the sub-α-gel phase while CIsubα equals 0.0 indicates that no sub-α-gel phase remains and the system is completely in the coagel phase.
The stability of the α-gel phase is affected by various internal and external factors. Incorporating negatively charged co-emulsifiers can increase the electrostatic repulsion between MG-bilayers and slow down the polymorphic transformation from the α-gel to the coagel phase.3,10,18 Sodium stearoyl lactylate (SSL) is a negatively charged molecule that naturally crystallizes into the α form.1 Previous studies have shown that adding 5–10% (w/w) SSL to GMS successfully improves the stability of the α-gel phase of a GMS–water hydrogel system.18 Other co-emulsifiers such as sodium stearate, DATEM, and phospholipids also improve the α-gel stability.10,15,19–22 However, co-emulsifiers are greatly affected by pH and ionic strength of the matrix.11,23,24 In terms of external factors, using slow cooling rates and avoiding shear upon and after cooling were found to also enhance the stability of the α-gel phase.8,18,25,26
Another strategy to enhance the stability of MG-structured emulsions is increasing the viscosity of the water phase using hydrocolloids, which improves the stability of the α-gel phase formed by MGs. Xanthan gum is a commonly used stabilizer in food and cosmetic emulsions because it forms a shear-thinning viscous fluid in both hot and cold water, and could increase the low-shear viscosity of the water phase at low concentrations.27,28 Additionally, xanthan gum is surface active at oil–water interfaces, which could help stabilize the emulsion by lowering the surface tension and introducing electrostatic repulsion between MG-bilayers.28,29
Previous work done by our group has investigated factors that affect the stability of MG–water systems. From a more practical point of view, most food and cosmetic systems structured with MGs are in the form of emulsions that contain both water and oil. This work will further examine various internal and external factors that could affect the long-term stability of MG-structured emulsions that contain 5% (w/w) and 15% (w/w) emulsifiers (i.e. GMS with SSL as the co-emulsifier) of high water content. The internal factors examined were the concentration of the co-emulsifiers and the addition of xanthan gum, while the external factors included cooling rate and shear upon cooling.
2. Experimental
2.1 Materials
The structured emulsions examined contained deionized water, oil, GMS, SSL, potassium sorbate and xanthan gum. The oil phase used in the emulsion was Neobee® M-5 oil from Stephan Company supplied by Charles Tennant & Company (Canada) Limited (Weston, ON, Canada). The GMS used was Alphadim 90 SBK provided by Caravan Ingredients (Lenexa, KS, USA) mainly contains GMS. The SSL used was Emplex Sodium Stearoyl Lactylate provided by Caravan Ingredients (Lenexa, Kansas, USA) and the xanthan gum was FASTir® Xanthan EC (TIC GUMS, White Marsh, MD, USA). The potassium sorbate was purchased from Sigma-Aldrich Canada Co. (Oakville, ON).2.2 Sample preparation
Emulsion samples with various GMS, SSL and xanthan gum concentrations studied in this work are listed in Table 1. The standard emulsions contained 5% (w/w) emulsifier, 70% (w/w) water and 25% (w/w) oil. In the water phase, 0.1% (w/w) potassium sorbate was added in all the samples while 0.07% (w/w) xanthan gum was added to selected samples. The oil phase was comprised of 25% (w/w) oil and 5% (w/w) emulsifiers at a 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) MG
1 (w/w) MG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SSL or 9
SSL or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) of MG
1 (w/w) of MG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SSL ratio. Samples with higher emulsifier concentration were prepared in order to improve on the signal to noise ratio of XRD signals, and these were called high-solids emulsions. The high-solids emulsions contained 60% (w/w) of water, 25% (w/w) of oil, and 15% (w/w) emulsifiers.
SSL ratio. Samples with higher emulsifier concentration were prepared in order to improve on the signal to noise ratio of XRD signals, and these were called high-solids emulsions. The high-solids emulsions contained 60% (w/w) of water, 25% (w/w) of oil, and 15% (w/w) emulsifiers.
| Water phase | Oil phase | ||||||
|---|---|---|---|---|---|---|---|
| Water | Potassium sorbate | Xanthan gum | Oil | GMS | SSL | ||
| Standard emulsion | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 SSL 19 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS GMS |
69.9 | 0.1 | 0 | 25 | 4.75 | 0.25 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 SSL 19 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum GMS with xanthan gum |
69.83 | 0.1 | 0.07 | 25 | 4.75 | 0.25 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 SSL 9 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS GMS |
69.9 | 0.1 | 0 | 25 | 4.5 | 0.5 | |
| High MG emulsion | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 SSL 19 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS GMS |
59.9 | 0.1 | 0 | 25 | 14.25 | 0.75 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 SSL 19 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum GMS with xanthan gum |
59.84 | 0.1 | 0.06 | 25 | 14.25 | 0.75 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 SSL 9 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS GMS |
59.9 | 0.1 | 0 | 25 | 13.5 | 1.5 | |
Emulsions were made using the following procedure. First, both the water phase and the oil phase were heated above the melting temperature of GMS (75 °C) in a microwave oven. The oil phase was then added into the water phase upon homogenization with a KitchenAid® two speed immersion blender (Whirlpool Corporation, St. Joseph, MI, USA) until a desired consistency was achieved. Emulsions for studying the effect of internal factors were quickly transferred to glass bottles and cooled to room temperature on the bench while emulsions for studying the effects of cooling rate and applied shear were cooled following the method used by Wang et al.18 Samples were prepared and analyzed in duplicate, and were stored at 45 °C in capped glass jars for accelerated shelf life tests.
2.3 Melting profiles
The melting profiles of the emulsions were examined with Mettler Thermal Analysis DSC 1 (Mettler Toledo Canada, Mississauga, Canada). Emulsion samples around 5–10 mg were weighted and hermetically sealed in aluminum pans and heated form 1 to 75 °C at 10 °C per minute. The melting profiles of the lotion samples were analyzed using Mettler's Stare software (Mettler Toledo Canada, Mississauga, ON, Canada). The CIsubα of MG-structured emulsions was calculated as described by Wang et al.182.4 Polymorphic forms
The lamellar spacing and polymorphic forms of the emulsions with high MG concentration were characterized with a Rigaku Multiflex X-ray Diffractometer (RigakuMSC Inc., The Woodlands, TX, USA). This unit has a copper source (λ = 1.54 Å) set at 44 kV and 40 mA, and the divergence slit, receiving slit, and scattering slit were set at 0.3 mm, 0.5°, and 0.5°, respectively. Emulsion samples were placed onto a glass sample holder with an area of 20 × 20 mm and depth of 1 mm. Samples were examined at diffraction angles in the range 1° < 2θ < 35° under the scanning rate of 1° per minute. Experiments were conducted at room temperature. The XRD results were analyzed with Jade 6.0 (Material Data Inc., Livermore, CA, USA).2.5 Water and oil mobility
The water and oil mobility in MG-structured emulsion was determined by pulsed proton nuclear magnetic resonance (NMR) using a Bruker mq20 Series NMR Analyzer (Bruker, Milton, ON, Canada). The 1H-NMR unit was connected to a circulating water bath set at 17 °C ± 0.5 °C to achieve 20 °C ± 0.5 °C in the measurement cell. Samples were prepared and the time constant from T2 relaxation was determined following methods published by Rogers et al.30 and Goldstein et al.25An inverse Laplace transform was applied to the free induction decays using CONTIN application along with Minispec software version 2.3 (Bruker, Milton, ON, Canada). The relative distribution of the number of protons relaxing at a given moment with their respective rate constants was computed with the CONTIN application. The median values of the relaxation times in each population were determined using PeakFit v4.12 (Systat Software Inc, San Jose, CA, USA) assuming a Gaussian distribution.
3. Results and discussion
3.1 CIsubα in MG-structured emulsions
Fig. 1 shows the melting and crystallization profiles of freshly prepared standard emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS. The two exothermic peaks (i.e., 1st and 2nd heating cycle) and the endothermic peak (i.e., cooling) at ∼15 °C, representing the phase transition between the sub-α-gel phase and the α-gel phase, displayed similar shape and area under the peak. On the other hand, the exothermic and endothermic peaks at ∼60 °C, representing the melting or crystallization of the α-gel phase, displayed different shapes and areas between the first heating cycle, the second heating cycle, and the crystallization cycle. The enthalpies of melting and crystallization of the sub-α-gel phase in the first heating, crystallization, and the second heating cycles were 1.24, 1.40, and 1.25 J g−1, while that of the α-gel gel phase were 4.55, 4.03, and 3.25 J g−1. When freshly prepared, the sub-α-gel phase and the α-gel phase should have the sample enthalpy of melting in the first and the second heating cycles because no coagel phase is formed. However, results only showed the same enthalpy of melting for the sub-α-gel phase but not for the α-gel phase. The difference in shape and area between the melting peaks of the α-gel phase obtained from the two heating cycles suggests that changes induced by heating may be affecting emulsion structure beyond a mere reversible change in polymorphic state. Furthermore, differences between the first and second heating cycle thermograms suggest that these changes are irreversible. As a result, changes in CI are due to both changes in emulsion structure as well as polymorphic form, while changes in CIsubα are strictly due to change in polymorphic form. Looking at the indicators used for the polymorphic transformation from the α-gel phase to the coagel phase in MG-structured emulsions, CI takes into account both the changes in emulsion structure and polymorphic form while CIsubα only takes that of the polymorphic form. Therefore CIsubα is a better indicator of the degree of the polymorphic transformation of MG-structured emulsions.
GMS. The two exothermic peaks (i.e., 1st and 2nd heating cycle) and the endothermic peak (i.e., cooling) at ∼15 °C, representing the phase transition between the sub-α-gel phase and the α-gel phase, displayed similar shape and area under the peak. On the other hand, the exothermic and endothermic peaks at ∼60 °C, representing the melting or crystallization of the α-gel phase, displayed different shapes and areas between the first heating cycle, the second heating cycle, and the crystallization cycle. The enthalpies of melting and crystallization of the sub-α-gel phase in the first heating, crystallization, and the second heating cycles were 1.24, 1.40, and 1.25 J g−1, while that of the α-gel gel phase were 4.55, 4.03, and 3.25 J g−1. When freshly prepared, the sub-α-gel phase and the α-gel phase should have the sample enthalpy of melting in the first and the second heating cycles because no coagel phase is formed. However, results only showed the same enthalpy of melting for the sub-α-gel phase but not for the α-gel phase. The difference in shape and area between the melting peaks of the α-gel phase obtained from the two heating cycles suggests that changes induced by heating may be affecting emulsion structure beyond a mere reversible change in polymorphic state. Furthermore, differences between the first and second heating cycle thermograms suggest that these changes are irreversible. As a result, changes in CI are due to both changes in emulsion structure as well as polymorphic form, while changes in CIsubα are strictly due to change in polymorphic form. Looking at the indicators used for the polymorphic transformation from the α-gel phase to the coagel phase in MG-structured emulsions, CI takes into account both the changes in emulsion structure and polymorphic form while CIsubα only takes that of the polymorphic form. Therefore CIsubα is a better indicator of the degree of the polymorphic transformation of MG-structured emulsions.
 | ||
Fig. 1 DSC melting and crystallization curves of freshly prepared standard emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL 19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS. GMS. | ||
3.2 Internal factors
Fig. 2(a–c) presents the melting profiles of high-solids emulsions when they were freshly made and after storage at 45 °C for four weeks. All samples showed two exothermic peaks at ∼15 °C and at 55–60 °C when freshly made, representing the phase transition from the sub-α-gel phase to the α-gel phase and the melting of the α-gel phase respectively. After storing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS emulsion for four weeks, the second melting peak was very broad, ranging from 52 to 65 °C, including three overlapping peaks (Fig. 2a). The observation of these overlapping peaks suggests possible phase separation or destabilization of the emulsion. On the other hand, the emulsion with higher SSL in GMS ratios (i.e., 1
GMS emulsion for four weeks, the second melting peak was very broad, ranging from 52 to 65 °C, including three overlapping peaks (Fig. 2a). The observation of these overlapping peaks suggests possible phase separation or destabilization of the emulsion. On the other hand, the emulsion with higher SSL in GMS ratios (i.e., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS) displayed only two melting peaks – a broad melting peak at ∼58 °C with an overlapping peak appearing at 60 °C after storage at 45 °C for four weeks (Fig. 2b). The melting profile of emulsions containing 1
GMS) displayed only two melting peaks – a broad melting peak at ∼58 °C with an overlapping peak appearing at 60 °C after storage at 45 °C for four weeks (Fig. 2b). The melting profile of emulsions containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum also showed a very broad melting peak including multiple peaks after four weeks of storage at 45 °C. Comparing the three emulsion formulations, one can notice that the sample with higher SSL content (1
GMS with xanthan gum also showed a very broad melting peak including multiple peaks after four weeks of storage at 45 °C. Comparing the three emulsion formulations, one can notice that the sample with higher SSL content (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS) showed similar melting curves after four weeks of incubation than when freshly prepared, indicating that it was structurally more stable.
GMS) showed similar melting curves after four weeks of incubation than when freshly prepared, indicating that it was structurally more stable.
The CIsubα for both high-solids emulsions (Fig. 2d) and standard emulsions (Fig. 2e) decreased and approached zero upon aging; however, CIsubα decreased slower in high-solids emulsions. Such decrease in CIsubα indicates that the polymorphic transformation from the sub-α-gel phase or the α-gel phase to the coagel phase in all the systems, and the polymorphic transformation were slowed down slightly by higher emulsifier (GMS and SSL) concentrations. Similar trends in the change of CIsubα suggest both the high-solids and standard samples transformed from the α-gel phase to the coagel phase. Therefore, to be able to obtain a higher signal to noise ratio, high-solids emulsions were examined with powder XRD.
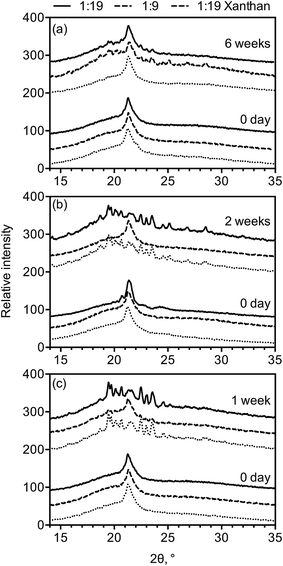
The XRD patterns of fresh high-solids' emulsions and after storage at 45 °C for four weeks are summarized in Fig. 3. The three formulations displayed similar SAXS peaks at 55 Å, 25 Å and 16 Å and WAXS peaks at 4.17 Å when freshly prepared (Fig. 3a and b), indicating they were all in the α-gel phase. After incubation at 45 °C for four weeks, samples with higher SSL content and with xanthan gum were in the α-gel phase (WAXS peak at 4.17 Å), while samples with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS transformed into the coagel phase (WAXS peaks between 3.6 and 4.6 Å) (Fig. 3d). Two peaks at 55 Å and 49 Å were observed from 1
GMS transformed into the coagel phase (WAXS peaks between 3.6 and 4.6 Å) (Fig. 3d). Two peaks at 55 Å and 49 Å were observed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 SSL
9 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS and 1
GMS and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 SSL
19 SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan emulsions (Fig. 3c), suggesting the co-existence of lamellar structures with two different thicknesses, specifically the α-gel phase and the emerging coagel phase upon aging. The broad peaks in the SAXS region, diffracted by these two emulsions, suggest that the GMS bilayers remained hydrated and less crystalline. Emulsions structured with 1
GMS with xanthan emulsions (Fig. 3c), suggesting the co-existence of lamellar structures with two different thicknesses, specifically the α-gel phase and the emerging coagel phase upon aging. The broad peaks in the SAXS region, diffracted by these two emulsions, suggest that the GMS bilayers remained hydrated and less crystalline. Emulsions structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS, on the other hand, displayed well-defined SAXS peaks at 49 Å, 24.5 Å, 16.5 Å, 12.4 Å and 8.3 Å (Fig. 3c), suggesting the presence of the more crystalline and less swollen lamellar structures compared with freshly prepared systems.31 In summary, results show that increasing SSL concentration and adding xanthan gum increases the water swelling capacity of lamellar structures formed by GMS, which in turn improves the stability of the α-gel phase of the structured emulsions.
GMS, on the other hand, displayed well-defined SAXS peaks at 49 Å, 24.5 Å, 16.5 Å, 12.4 Å and 8.3 Å (Fig. 3c), suggesting the presence of the more crystalline and less swollen lamellar structures compared with freshly prepared systems.31 In summary, results show that increasing SSL concentration and adding xanthan gum increases the water swelling capacity of lamellar structures formed by GMS, which in turn improves the stability of the α-gel phase of the structured emulsions.
 | ||
| Fig. 3 XRD patterns of high-solid emulsions. (a) SAXS and (b) WAXS of freshly prepared samples, and (c) SAXS and (d) WAXS of samples stored at 45 °C for four weeks. | ||
The distributions of pulse NMR T2 relaxation times are summarized in Fig. 4. The time constants of the primary components of MG-structured emulsions, water and oil, were determined. The measured T2 time constant for water was 2479 ms, in agreement with the literature reported value,25 and that for Neobee® oil were 48, 104, and 215 ms. No time constant that is correlated with the presence of free water was observed in any of the MG-structured emulsions, indicating that water in all the emulsions was bound within the multi-lamellar structure and thus displayed restricted mobility. The relaxation distribution of the standard emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS shifted to higher time constant after one week and four weeks (Fig. 4a), indicating increased mobility and decreased water and oil binding. The sample structured with 1
GMS shifted to higher time constant after one week and four weeks (Fig. 4a), indicating increased mobility and decreased water and oil binding. The sample structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS displayed a narrower distribution of T2 relaxation times, but were also shifted to longer times upon aging (Fig. 4b). Higher T2 relaxation time was observed from samples structured with 1
GMS displayed a narrower distribution of T2 relaxation times, but were also shifted to longer times upon aging (Fig. 4b). Higher T2 relaxation time was observed from samples structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum at day 0 (Fig. 4c), this indicates that the addition of xanthan gum initially leads to higher water and oil mobility than the other two formulations. However, throughout storage for four weeks, only small shifts in the distribution of relaxation times were observed, indicating that xanthan gum helps increase the stability of the structured emulsion. The relaxation distributions of high-solids emulsions are not presented because their distribution of the three populations was similar with those of the standard emulsions.
GMS with xanthan gum at day 0 (Fig. 4c), this indicates that the addition of xanthan gum initially leads to higher water and oil mobility than the other two formulations. However, throughout storage for four weeks, only small shifts in the distribution of relaxation times were observed, indicating that xanthan gum helps increase the stability of the structured emulsion. The relaxation distributions of high-solids emulsions are not presented because their distribution of the three populations was similar with those of the standard emulsions.
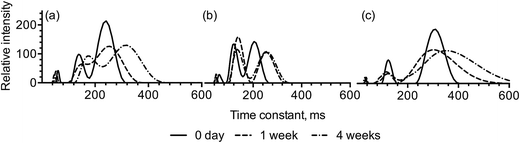 | ||
Fig. 4 T2 relaxation distribution of standard emulsions structured with (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL 19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS, (b) 1 GMS, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL 9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS, and (c) 1 GMS, and (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL 19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum. GMS with xanthan gum. | ||
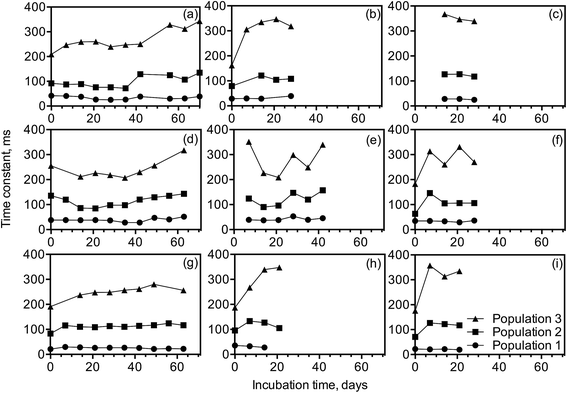
The computed median values of the time constants of standard and high-solids emulsions are presented in Fig. 5. The relaxation distribution of the standard emulsions that contain 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS and with xanthan gum showed less of a T2 increase in time for population 3 compared with the emulsion structured with less GMS without xanthan (i.e. 1
GMS and with xanthan gum showed less of a T2 increase in time for population 3 compared with the emulsion structured with less GMS without xanthan (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS) (Fig. 5a–c). This therefore shows that the presence of xanthan gum or higher SSL content in the structured emulsions results in lower water and oil mobility and stronger binding. Compared to standard emulsions, high-solids systems (Fig. 5d–f) displayed lower time constants because these systems contain less liquid, but they showed a similar increasing trend upon aging.
GMS) (Fig. 5a–c). This therefore shows that the presence of xanthan gum or higher SSL content in the structured emulsions results in lower water and oil mobility and stronger binding. Compared to standard emulsions, high-solids systems (Fig. 5d–f) displayed lower time constants because these systems contain less liquid, but they showed a similar increasing trend upon aging.
Higher solids content increased the overall water and oil-binding capacity of the lamellar structure formed by GMS and decreased T2 relaxation times, however the water and oil mobility in these systems also increased upon aging. This increase in water and oil mobility in GMS-structured emulsions is caused by micro scale release of water due to the polymorphic transformation from the α-gel phase to the coagel phase.
Results suggest that internal factors which improve the stability of the α-gel phase in MG-structured emulsion are similar to those that improve the stability of the α-gel phase of MG–water systems.18 Increasing the concentration of α-tending co-emulsifiers (SSL) and adding xanthan gum increased the α-gel stability of MG-structured emulsions by increasing water layer thickness in the lamellar structure and enhancing water and oil binding capacity in the structured emulsions.
3.3 External factors
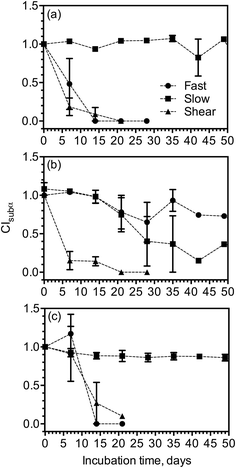
Fast cooling rate, slow cooling rate, and cooling with shear were examined as external factors that potentially affect the stability of MG-structured emulsions. As previously discussed, the standard and high-solids samples displayed similar trends in changes that occur in their structural and polymorphic properties, only CIsubα for high-solids samples are presented in Fig. 6. All the formulations displayed a large decrease in CIsubα from 1.0 to 0.0 within two weeks when cooled under shear during preparation, indicating that shear leads to faster polymorphic transition from the α-gel phase to the coagel phase in MG-structured emulsions. Emulsions structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS, with and without xanthan gum (Fig. 6a and c), both maintained a CIsubα of ∼1.0 for longer than six weeks when cooled at a slow cooling rate during preparation, while their CIsubα decreased to 0.0 in three weeks when cooled at a fast rate during preparation. Slow cooling therefore allowed the formation of more stable sub-α-gel phase and α-gel phase in these MG-structured emulsions. The emulsion structured with 1
GMS, with and without xanthan gum (Fig. 6a and c), both maintained a CIsubα of ∼1.0 for longer than six weeks when cooled at a slow cooling rate during preparation, while their CIsubα decreased to 0.0 in three weeks when cooled at a fast rate during preparation. Slow cooling therefore allowed the formation of more stable sub-α-gel phase and α-gel phase in these MG-structured emulsions. The emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS (Fig. 6b) showed a similar decrease in CIsubα under fast and slow cooling rates but CIsubα decreased slowly to 0.0 under a fast cooling rate after six weeks.
GMS (Fig. 6b) showed a similar decrease in CIsubα under fast and slow cooling rates but CIsubα decreased slowly to 0.0 under a fast cooling rate after six weeks.
WAXS patterns of high-solids emulsions prepared using a slow cooling rate, a fast cooling rate, in the presence and absence of shear are compared in Fig. 7. All emulsions were in the α-gel phase when freshly prepared, characterized by a single WAXS spacing at 4.17 Å. Emulsions structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with and without xanthan gum under slow cooling rate preserved the α-gel phase for up to six weeks, characterized by single WAXS peak at 4.17 Å in Fig. 7a. The emulsion structured with 1
GMS with and without xanthan gum under slow cooling rate preserved the α-gel phase for up to six weeks, characterized by single WAXS peak at 4.17 Å in Fig. 7a. The emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS diffracted a major WAXS peak at 4.17 Å with minor peaks between 3.6 and 4.6 Å after six weeks, suggesting that the system had started to transform into the coagel phase. Under fast cooling rate (Fig. 7b), only the emulsion structured with 1
GMS diffracted a major WAXS peak at 4.17 Å with minor peaks between 3.6 and 4.6 Å after six weeks, suggesting that the system had started to transform into the coagel phase. Under fast cooling rate (Fig. 7b), only the emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS displayed a WAXS peak representing the α-gel phase at 4.17 Å, while the other two samples were already in the coagel phase, indicated by peaks between 3.6 and 4.6 Å. After one week of storage, all samples cooled under shear displayed peaks between 3.6 and 4.6 Å (Fig. 7c), and only emulsions structured with higher SSL content (i.e. 1
GMS displayed a WAXS peak representing the α-gel phase at 4.17 Å, while the other two samples were already in the coagel phase, indicated by peaks between 3.6 and 4.6 Å. After one week of storage, all samples cooled under shear displayed peaks between 3.6 and 4.6 Å (Fig. 7c), and only emulsions structured with higher SSL content (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS) still displayed a small peak at 4.17 Å. Applied shear upon cooling therefore accelerated the formation of the coagel phase, in agreement with results obtained from DSC experiments.
GMS) still displayed a small peak at 4.17 Å. Applied shear upon cooling therefore accelerated the formation of the coagel phase, in agreement with results obtained from DSC experiments.
 | ||
| Fig. 7 WAXS patterns of high-solid emulsions processed (a) under slow and (b) fast cooling rates, and (c) cooled with shear. | ||
The T2 relaxation times of MG-structured emulsions prepared under various cooling conditions and incubated at 45 °C are presented in Fig. 8. No further time points were measured after the CIsubα decreased to 0.0 (i.e., when the system was completely transformed into the coagel phase). For all the formulations, samples prepared under slow cooling rates showed smaller T2 relaxation times compared to those prepared under high cooling rates and under shear. This therefore indicates that the water and oil had less mobility and were highly bound within the multi-lamellar structured when prepared under slow cooling rates compared with prepared using fast cooling rates or shear. The emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with xanthan gum showed the smallest increase in T2 relaxation times after 60 days among the three formulations (Fig. 8g), and was therefore considered the most stable. On the other hand, the emulsion structured with 1
GMS with xanthan gum showed the smallest increase in T2 relaxation times after 60 days among the three formulations (Fig. 8g), and was therefore considered the most stable. On the other hand, the emulsion structured with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (w/w) SSL
9 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS had a smaller T2 relaxation time when prepared using a fast cooling rate (Fig. 8e) or under shear relative to the other two formulations (Fig. 8f). This suggests that higher SSL levels improved the emulsion's stability towards higher cooling rate and shear.
GMS had a smaller T2 relaxation time when prepared using a fast cooling rate (Fig. 8e) or under shear relative to the other two formulations (Fig. 8f). This suggests that higher SSL levels improved the emulsion's stability towards higher cooling rate and shear.
Results suggested that slow cooling rates promoted the formation of a more stable α-gel phase while fast cooling rate and applied shear accelerated the polymorphic transformation from the α-gel phase to the coagel phase in GMS-structured emulsions. This is in agreement with previous studies on GMS–water hydrogels and MG-structured emulsions.8,18,25,26 Even though previous work showed that slow cooling rates promote the formation of larger droplets,8 higher α-gel stability was still achieved in emulsions with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 (w/w) SSL
19 (w/w) SSL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GMS with or without xanthan gum under slow cooling rates. One possible explanation to such phenomenon is that slow cooling rates provide MG molecules with more time to mix with co-emulsifiers, oil, and water to self-assemble into fully hydrated lamellar structures.32 On the other hand, fast cooling rates and the application of shear upon cooling promote the nucleation into the coagel phase and disrupt the formation of continuous lamellar hydrate, resulting in the enhancement of water release from the multi-lamellar structure.18 Additionally, the behaviour of the emulsion systems under different cooling conditions was affected by composition as it was found that higher SSL content possibly increased the emulsions' stability against higher cooling rates and shear.
GMS with or without xanthan gum under slow cooling rates. One possible explanation to such phenomenon is that slow cooling rates provide MG molecules with more time to mix with co-emulsifiers, oil, and water to self-assemble into fully hydrated lamellar structures.32 On the other hand, fast cooling rates and the application of shear upon cooling promote the nucleation into the coagel phase and disrupt the formation of continuous lamellar hydrate, resulting in the enhancement of water release from the multi-lamellar structure.18 Additionally, the behaviour of the emulsion systems under different cooling conditions was affected by composition as it was found that higher SSL content possibly increased the emulsions' stability against higher cooling rates and shear.
4. Conclusion
This work examined internal and external factors that affect the polymorphic transformation and stability of GMS-structured o/w emulsions. The sub-α Coagel Index was used for the first time as an effective parameter to characterize the degree of coagel formation in MG-structured emulsions.The internal and external factors examined in this work were shown to affect the polymorphic form and stability of GMS-structured emulsions in complex ways. Increasing the concentration of α-tending co-emulsifiers, specifically SSL, improved the stability of GMS-structured emulsions. The addition of xanthan gum also improved emulsion stability and the mechanism possibly involved enhancing electrostatic repulsion between GMS lamellae and increasing the viscosity of the water phase. Higher emulsion stability was also obtained when the emulsion was formed using a slow cooling rate, while cooling under shear was found to decrease emulsion stability. Increasing SSL concentration improved emulsion stability against faster cooling rates and shear.
The effects of internal and external factors on the stability of the α-gel phase in MG-structured o/w emulsions were similar to their effects on MG–water systems. Understanding the nature and dynamics of simple MG–water systems thus successfully predicted the behaviour in more complex MG-structured emulsion systems with high water content.
Acknowledgements
This work was funded by Natural Sciences and Engineering Research Council of Canada. The authors would like to thank Ms Karissa Salama-Frakes for her help with NMR data processing.References
- N. J. Krog, in Food Emulsions, ed. S. E. Friberg and K. Larsson, Marcel Dekker, New York, 3rd edn, 1997 Search PubMed.
- A. G. Marangoni and S. H. J. Idziak, US Pat. 7,718,210 B2, 2010.
- A. G. Marangoni, S. H. J. Idziak, C. Vega, H. Batte, M. Ollivon, P. S. Jantzi and J. W. E. Rush, Soft Matter, 2007, 3, 183 RSC.
- H. D. Batte, A. J. Wright, J. W. Rush, S. H. J. Idziak and A. G. Marangoni, Food Biophys., 2007, 2, 29–37 CrossRef.
- A. Goldstein and K. Seetharaman, Food Res. Int., 2011, 44, 1476–1481 CrossRef CAS.
- S. Calligaris, L. Manzocco, F. Valoppi and M. C. Nicoli, Food Res. Int., 2013, 51, 596–602 CrossRef CAS.
- A. I. Blake and A. G. Marangoni, Food Res. Int., 2015, 74, 284–293 CrossRef CAS.
- H. D. Batte, A. J. Wright, J. W. Rush, S. H. J. Idziak and A. G. Marangoni, Food Res. Int., 2007, 40, 982–988 CrossRef CAS.
- N. K. Ojijo, I. Neeman, S. Eger and E. Shimoni, J. Sci. Food Agric., 2004, 84, 1585–1593 CrossRef CAS.
- I. Heertje, E. Roijers and H. A. C. Hendrickx, LWT--Food Sci. Technol., 1998, 31, 387–396 CrossRef CAS.
- N. Krog and K. Larsson, Chem. Phys. Lipids, 1968, 2, 129–143 CrossRef CAS PubMed.
- K. Larsson and N. Krog, Chem. Phys. Lipids, 1973, 10, 177–180 CrossRef CAS.
- N. Krog and A. P. Borup, J. Sci. Food Agric., 1973, 24, 691–701 CrossRef CAS.
- K. Larsson, K. Fontell and N. Krog, Chem. Phys. Lipids, 1980, 27, 321–328 CrossRef CAS.
- A. Zetzl, M. Ollivon and A. Marangoni, Cryst. Growth Des., 2009, 9, 3928–3933 CAS.
- F. C. Wang and A. Marangoni, RSC Adv., 2014, 4, 50417–50425 RSC.
- G. Cassin, C. de Costa, J. P. M. van Duynhoven and W. G. M. Agterof, Langmuir, 1998, 14, 5757–5763 CrossRef CAS.
- F. C. Wang and A. Marangoni, RSC Adv., 2015, 5, 43121–43129 RSC.
- M. B. Munk, A. G. Marangoni, H. K. Ludvigsen, V. Norn, J. C. Knudsen, J. Risbo, R. Ipsen and M. L. Andersen, Food Res. Int., 2013, 54, 1738–1745 CrossRef CAS.
- A. Sein, J. A. Verheij and W. G. M. Agterof, J. Colloid Interface Sci., 2002, 249, 412–422 CrossRef CAS PubMed.
- J. P. M. van Duynhoven, I. Broekmann, A. Sein, G. M. P. van Kempen, G.-J. W. Goudappel and W. S. Veeman, J. Colloid Interface Sci., 2005, 285, 703–710 CrossRef CAS PubMed.
- L. Rydhag and I. Wilton, J. Am. Oil Chem. Soc., 1981, 58, 830–837 CrossRef CAS.
- L. Mao, S. Calligaris, L. Barba and S. Miao, Food Res. Int., 2014, 58, 81–88 CrossRef CAS.
- F. C. Wang and A. G. Marangoni, RSC Adv., 2015 Search PubMed , submitted.
- A. Goldstein, A. Marangoni and K. Seetharaman, Food Biophys., 2012, 7, 227–235 CrossRef.
- S. Da Pieve, S. Calligaris, E. Co, M. C. Nicoli and A. G. Marangoni, Food Biophys., 2010, 5, 211–217 CrossRef.
- E. Dickinson, J. Sci. Food Agric., 2013, 93, 710–721 CrossRef CAS PubMed.
- M. Izydorczyk, S. W. Cui and Q. Wang, in Food Carbohydrates – Chemistry, Physical Properties, and Applications, CRC Press, Boca Raton, Florida, 2005 Search PubMed.
- M. Hennock, R. R. Rahalkar and P. Richmond, J. Food Sci., 1984, 49, 1271–1274 CrossRef.
- M. A. Rogers, A. J. Wright and A. G. Marangoni, Soft Matter, 2008, 4, 1483–1490 RSC.
- F. Peyronel and R. Campos, in Structure-Function Analysis of Edible Fats, ed. A. G. Marangoni, AOCS Press, Urbana, 2012, pp. 235–294 Search PubMed.
- J. F. Toro-vazquez, E. Dibildox-alvarado and V. Herrera-coronado, in Crystallization and Solidification Properties of Lipids, ed. N. Widlak, R. Hartel and S. Narie, AOCS Press, Champaign, Illinois, 2001, pp. 53–78 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |