α-Bi2O3 photoanode in DSSC and study of the electrode–electrolyte interface†
Abstract
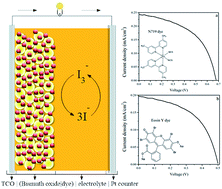
One of the many interfaces in Dye Sensitized Solar Cells (DSSC) which affects the performance of the cell is the photoanode–electrolyte interface. Material selection, its morphology, size and surface properties are found to control the electron transfer and transport behaviour of the photoanode. Here α-bismuth oxide (α-Bi2O3) is chosen as a photoanode material, which is synthesized by an easy and cost effective method, citrate nitrate gel combustion. Basic and specific analyses to study the properties of this material to use as a photoanode is done by using, X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), UV-visible spectroscopy, Hall measurement and Electrochemical Impedance Spectroscopy (EIS). Since the electron transfer dynamics between photoanode–electrolyte interfaces have a significant impact on the performance efficiency of the cell, a detailed electrochemical impedance analysis is done by using bismuth oxide as the photoanode. Two different dyes, an organometallic dye (N719) and an organic dye (Eosin Y), are used as light harvesters in the cell structure with an iodine/iodide electrolyte. The performance efficiency of the photoanode is evaluated by recording the I–V characteristic of the cell under 1 sun illumination. The efficiency values obtained for dyes N719 and Eosin Y are 0.09 and 0.05% respectively and this is correlated with the impedance data obtained. Bode and Nyquist plots are used to explain the obtained results. Though nano bismuth oxide prepared by this synthetic method shows good charge carrier concentration, mobility and conductivity, the efficiency of the cell with the bismuth oxide photoanode is low. This low efficiency is attributed to the poor dye attachment and back recombination at the photoanode–electrolyte interface. Interface impedance data supports this argument and suggests modification of the oxide structure, morphology and surface properties for the improvement of efficiency.

 Please wait while we load your content...
Please wait while we load your content...