Hyaluronic acid-mediated one-pot facile synthesis of a sensitive and biocompatible Gd2O3 nanoprobe for MR imaging in vivo†
Haoyu Wangb,
Yan-Yan Fua,
Xuejun Zhanga,
Chunshui Yu*ab and
Shao-Kai Sun*a
aSchool of Medical Imaging, Tianjin Medical University, Tianjin 300203, China. E-mail: shaokaisun@163.com
bDepartment of Radiology, Tianjin Key Laboratory of Functional Imaging, Tianjin Medical University General Hospital, Tianjin 300052, China. E-mail: chunshuiyu@tijmu.edu.cn
First published on 6th October 2015
Abstract
MR contrast agents play a crucial role in the early diagnosis of various diseases, and many nanoprobes with versatile properties have been developed. However, in order to seek high sensitivity, the biocompatibility and systemic toxicity assessment of the nanoprobes have attracted much less attention. To meet clinical requirements, it is highly desirable to develop a highly sensitive and biocompatible nanoprobe using a facile procedure. Herein, we report a sensitive and biocompatible Gd2O3 nanoprobe using hyaluronic acid (HA) via a one-pot facile synthesis. HA not only endows the nanoprobe with excellent biocompatibility, but also gives a synergistic effect for improving the sensitivity of the nanoprobe in the aspects of increasing rotational correlation time, water exchange rate and the ratio of surface Gd3+ to the inner ones. What’s more, the nanoprobe has specific ability for adrenal gland MR imaging. These results suggest that the HA–Gd2O3 nanoprobe has a potentially promising application in MR imaging in vivo.
Introduction
Magnetic resonance imaging (MRI) as a non-invasive technique plays an important role in fundamental research and clinical diagnosis, which possesses the merits of high spatial resolution, lack of ionizing radiation, abundant physiological and anatomical information, and a remarkable capacity for differentiating soft tissues. To increase the signal contrast between the lesion and normal tissue, MR contrast agents have been widely used in clinical diagnosis.1,2 As Gd-based T1 contrast agents give bright images, which favor the accurate diagnosis of various diseases,3,4 Gd-chelates are the most commonly used T1-contrast agents in clinics, owing to their advantages of high thermodynamic and kinetic stability, and relatively low toxicity, however, they suffer from low r1 values, short blood circulation times, and low specificity to organs or tissues.3,4With the explosive development of nanotechnology, many nanoprobes with excellent contrast effects, various sizes and shapes, versatile surface modification and long blood circulation times have been fabricated for application in the fields of blood pool imaging, organ and tumor-targeted imaging, highly promoting the early diagnosis of various diseases.5,6 To meet the requirements of clinical diagnosis, the safety of highly efficient nanoprobes needs to be considered before application to clinical diagnosis.7,8 The potential toxicity in vivo seriously limited further development in the field of translational medicine.9,10 The following aspects should be considered for the design of an ideal Gd-based MR contrast: (1) high sensitivity of the contrast agent is essential to distinguish normal and diseased tissues and show enough significant enhancement in imaging time; (2) the contrast agent should possess good biocompatibility and show low toxicity in vivo; (3) the developed MR contrast agent should possess a passive or active targeting ability to accumulate at the site of lesion or target organ; (4) facile synthesis and mild conditions are also crucial for large-scale industrial production.3 It is very attractive and highly desirable to develop an innovative strategy to fabricate T1 MRI contrast agents with the above-mentioned properties.
To improve the sensitivity of T1 MRI contrast agents, the parameters that influence relaxivity should be optimized comprehensively.11 According to the Solomon–Bloembergen–Morgan (SBM) theory,12 T1 relaxation originates from the interaction between the surrounding water including inner-sphere, second-sphere and outer-sphere water molecules with the contrast agent.13 The r1 value is governed by several factors, including the number of inner sphere water molecules (q), rotational tumbling time (τR) and water exchange rate (WER).14 For nanoparticles, water accessible Gd3+ ions at the crystal surface play a crucial role in the contribution to longitudinal relaxation of water protons, while bulk Gd3+ ions in the crystal lattice don’t due to the greatly lowered (or lost) water accessibility of the shielded Gd3+ ions. The ratio of surface Gd3+ to the inner ones (ratio) could highly influence the relaxivity of the nanoprobes.15,16 These factors should be considered when developing responsive and highly sensitive T1 contrast agents.
How to improve biocompatibility is attracting increasing attention in the development of nanoprobes for the diagnosis of various diseases.7–10 Surface modification via biocompatible macromolecules (dextran, chitosan and polyethylene glycol) is the most effective strategy to facilitate the nanoprobe biocompatibilty.3,17 However, the post-modification possesses disadvantages such as a tedious synthetic procedure, aggregation of the nanoparticles and poor water solubility. Recently, a mimicking biomineralization processes to fabricate biocompatible nanomaterials using biomacromolecules as the template had been extensively investigated. The mild conditions and high efficiency make the strategy attractive in the field of nanomedicine.18 Typically, fluorescent gold nanoclusters,19 silver nanoclusters,20 ZnS quantum dot,21 Gd2O3 (ref. 22) and Gd2O3/Au nanoparticles23 have been synthesized using albumin as template. Special sequence DNA was used to prepare silver nanoclusters with various fluorescent emission wavelengths.24 These nanomaterials as powerful probes have been applied in the field of biosensor and bioimaging.
Based on the goal of enhancing T1 contrast effect and biosynthetic strategy to improve the biocompatibility, we aimed to develop a sensitive and biocompatible Gd2O3 nanoprobe using hyaluronic acid (HA) via the mimicking biomineralization processes (Scheme 1). The reasons for using HA as the template were as follows: firstly, HA is a glycosaminoglycan which distributes widely throughout the body with good biocompatibility and excellent aqueous solubility.25 In addition, the HA–Gd2O3 nanoprobe as a slow-tumbling nano-scale contrast agent could increase τR and enhance the relaxivity.12,14 Last but not least, HA coated on a Gd2O3 core possesses a strong ability to retain water molecules, which could increase the water exchange rate effectively,26,27 and control the growth of Gd2O3 crystals of small size, which possess abundant surface Gd3+ ions in one nanoparticle, further increasing the ratio of surface Gd3+ to inner Gd3+ ions in the crystal lattice.15,16 The synergistic effect highly facilitates the sensitivity of the proposed nanoprobe. In general, HA not only endows the nanoprobe with excellent biocompatibility, but also gives a synergistic effect for improving the sensitivity of the nanoprobe.
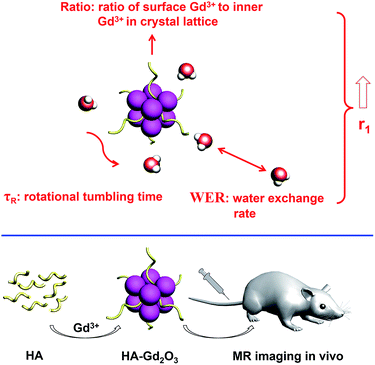 | ||
| Scheme 1 Schematic representation of hyaluronic acid-mediated synthesis of a Gd2O3 nanoprobe for MR imaging in vivo. | ||
In our current work, we use HA as a template to load Gd3+ and synthesise a highly sensitive HA–Gd2O3 nanoprobe with good biocompatibility by a facile one-pot reaction at room temperature. The nanoparticles were characterized with different methods and their MR imaging was evaluated in vitro and in vivo compared with Gd–DTPA. This new contrast agent has higher T1 values and brighter organ contrast enhancement. Moreover, it shows a targeting ability for adrenal gland imaging. The proposed HA–Gd2O3 nanoprobe possesses the advantages of high sensitivity, good biocompatibility, low dose in practice, and a facile preparation process, which shows great potential in the large-scale industrial production and further application in clinical medicine.
Materials and methods
Materials
HA (sodium salt, Mw ≈ 5000) was purchased from Bloomage Freda Biopharm Co., Ltd. (Jinan, China). Gd(NO3)3·6H2O and NaOH were obtained from Aladdin Chemistry Corporation. The deionized water used throughout the experiments was provided by Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China). Cell cultures including RPMI 1640, and fetal calf serum were obtained from Zhaoran Biotechnology Co., Ltd. (Tianjin, China). Kunming mice were purchased from Beijing HFK bioscience Co., Ltd. (Beijing, China) and fed in the SPF animal house. All chemicals and reagents were of analytical grade.Characterization
The morphology and microstructure were characterized by a Philips Tecnai G2 F20 (Philips, Eindhoven, The Netherlands) field emission high-resolution transmission electron spectroscopy (HRTEM). The samples for HRTEM were prepared by using a 230-mesh Cu grid coated with a lacey carbon film and loaded drying sample droplets from water dispersion onto it. The X-ray diffraction (XRD) spectra were collected on a Rigaku D/max-2500 X-ray diffractometer (Rigaku, Tokyo, Japan) with Cu Kα radiation. A Nicolet IR AVATAR-360 spectrometer (Nicolet, USA) with pure KBr as the background were used to measure the Fourier transform infrared (FT-IR) spectra (of 400–4000 cm−1). The measurements of dynamic light scattering (DLS) and zeta potential of HA–Gd2O3 nanoprobe were analysed by Malvern Zetasizer Nano ZS model ZEN3600 (standard 633 nm laser, Worcestershire, U.K.).Synthesis of HA–Gd2O3 nanoprobe
0.2 g of HA was dissolved in 10 mL of water, then 1 mL of Gd(NO3)3·6H2O (0.1 M) was added to the above solution under stirring. After 5 min, 0.6 mL of NaOH (1 M) was added to the mixture to form an alkaline environment (pH ≈ 10). After vigorously stirring for 1 h at room temperature, the solution was dialysed for 24 h against an excess of water to remove unreacted reagents, followed by freeze-drying to yield the purified product as a white flocculent solid.T1 relaxivity and T1-weighted MR imaging in vitro
Different concentrations of HA–Gd2O3 nanoprobe and Gd–DTPA were prepared in a series of Gd3+ concentrations ranging from 0.025 to 1.0 mM in 1.5 mL tubes, respectively. The T1 relaxivity (r1) and T1-weighted MR images of HA–Gd2O3 compared with Gd–DTPA were obtained by a 0.5 T MesoMR60 (Shanghai Niumag Corporation, China) with the following parameters: multi spin-echo, TR/TE = 2000/60 ms, FOV of 100 × 100 mm, slices = 1 and matrix of 192 × 256.Stability measurements
HA–Gd2O3 nanoprobe (8 mg mL−1) was dissolved in normal saline and stored at 4 °C for 1 day and 30 days. Then DLS measurement was performed to investigate the colloid stability of the nanoprobe.Cytotoxicity assay in vitro
Methyl thiazolyl tetrazolium (MTT) assay was used to assess the in vitro cytotoxicity of the nanoprobe. Briefly, a human cervical epidermal carcinoma cell line (HeLa cell) was previously cultured in RPMI-1640 mixed with 10% fetal bovine serum and 1% penicillin–streptomycin. Then the cells were seeded into a 96-well cell culture plate at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well. After incubation for 24 h at 37 °C under 5% CO2, different concentrations of HA–Gd2O3 nanoprobes dissolved in normal saline were added to the wells. The cells were continuously cultured at 37 °C under 5% CO2. After another 24 h, MTT (10 μL, 5 mg mL−1) was added to each well and incubated at 37 °C for 4 h. Finally, the upper solution was removed and 120 μL DMSO was added to dissolve the formazan crystal. The optical density was measured at 490 nm by a microplate reader (Bio-tek). The viability of cell growth was calculated by the following equation: cell viability (%) = (mean of sample group/mean of control) × 100%.
000 per well. After incubation for 24 h at 37 °C under 5% CO2, different concentrations of HA–Gd2O3 nanoprobes dissolved in normal saline were added to the wells. The cells were continuously cultured at 37 °C under 5% CO2. After another 24 h, MTT (10 μL, 5 mg mL−1) was added to each well and incubated at 37 °C for 4 h. Finally, the upper solution was removed and 120 μL DMSO was added to dissolve the formazan crystal. The optical density was measured at 490 nm by a microplate reader (Bio-tek). The viability of cell growth was calculated by the following equation: cell viability (%) = (mean of sample group/mean of control) × 100%.
Cell uptake in vitro
The cell uptake assay was carried out using HeLa cells, which were seeded into a 96-well cell culture plate at a density of 5 × 105 per well. Different masses of HA–Gd2O3 nanoprobes (25 μg, 125 μg) were added into the corresponding wells (n = 6) after incubating for 24 h at 37 °C under 5% CO2. After another 24 h, the extracellular media was removed and the cells were washed with PBS (0.1 mM) twice. These fluids were collected together as extracellular medium. Then the cells were digested by pancreatin and also washed with PBS twice to obtain intracellular fluid. The collected fluid was dissolved by aqua regia and quantified by ICP-MS to assess the percentage of Gd element by cell uptake.Biodistribution of the nanoprobe
To assess the tissue distribution of the HA–Gd2O3 nanoprobe, Kunming mice were treated with HA–Gd2O3 (0.025 mmol Gd per kg). Then the mice were sacrificed and the major organs (heart, liver, spleen, lung, kidney and adrenal gland) were collected at various times (2 days, 15 days, 30 days, n = 3 for each time point). These organs were dissolved by aqua regia for a week and then the Gd contents were analysed by ICP-MS.Toxicity assay in vivo
All the animal studies were conducted with the approval of the Tianjin Medical University Animal Care and Use Committee. Kunming mice (24–26 g) and HA–Gd2O3 nanoprobe dissolved in normal saline were prepared for assay. (a) Weight change: Kunming mice with intravenously injected with 0.2 mL HA–Gd2O3 saline solution (0.025 mmol Gd per kg) and normal saline were regarded as the test and control group, respectively (n = 3 for each group). The body weight were monitored for a month. (b) Histology analysis: after intravenous injection of HA–Gd2O3 (200 μL, 0.025 mmol Gd per kg) as the test group and normal saline (200 μL) as the control group, the mice were sacrificed at different time points (1, 7 and 30 days) and the major organs (including heat, liver, spleen, kidney and adrenal gland) were extracted. These organs were kept in formaldehyde (4%) and stained with hematoxylin and eosin (H&E) to investigate the histological toxicity of the nanoprobe. (c) Blood biochemistry assessment: blood was harvested from mice treated with HA–Gd2O3 (200 μL, 0.025 mmol Gd per kg) as the test group and without injection as the control group (n = 3 for each group) at different times (1 and 7 days). Liver function markers including total protein (TP), albumin (ALB), globulin (GLB), alanine aminotransferase (ALT), gamma glutamyl transaminase (GGT), aspartate aminotransferase (AST), and kidney function indicators including urea (UREA) and creatinine (CREA) were analyzed at the clinical laboratory in Tianjin Medical University General Hospital.MR imaging in vivo
MR imaging of mice before and after intravenous injection of 200 μL of HA–Gd2O3 (0.025 mmol Gd per kg), Gd–DTPA (0.025 mmol Gd per kg) and Gd–DTPA (0.1 mmol Gd per kg), respectively, was performed on a 3.0 T clinical MR system with a small animal receiver coil (GE Signa Excite). The mice were anesthetized by 4% chloral hydrate (6 mL kg−1) and MR Images were obtained with a series of timed post injections of contrast agents using a fast spin-echo sequence with the following parameters: (TR/TE = 360/10 ms; FA = 111°; FOV = 100 mm × 100 mm; slice thickness = 3 mm without gap; 7 coronal slices obtained).Results and discussion
Synthesis and characterization
To obtain the HA–Gd2O3 nanoprobe with high Gd content and good colloid stability, the dosage of Gd3+ was carefully optimized. Typically, 1 mL of different concentrations of Gd(NO3)3 (0.02 M, 0.05 M, 0.1 M, 0.2 M, 0.5 M) was added respectively to 10 mL of HA aqueous solution (20 mg mL−1) and then NaOH was introduced for the reaction to proceed in an alkaline environment (pH ≈ 10). As shown in Fig. S1,† precipitation began to form as the concentration of Gd3+ increased to 0.2 M, which indicated HA could not stabilize the Gd2O3 nanoparticles efficiently. So 0.1 M was chosen to be the optimal reaction concentration to meet the requirements of high Gd content and good colloid stability.The prepared HA–Gd2O3 nanoprobe was characterized by HRTEM, DLS, FL-IR, XRD and XPS. HRTEM showed the morphological shape of the nanoprobe was spherical and the mean diameter was about 2 nm (Fig. 1a and S2†). As strong hydrogen bond formation occurred between the water molecules and adjacent carboxyl and N-acetyl groups of HA, the hydrodynamic diameter originating from the assembled nanoprobes was much larger than the particle diameter (2 nm) estimated from HRTEM.28 The zeta potential of the nanoprobe dispersed in water is −15.5 mV. The FT-IR spectra showed that the characteristic bands of pure HA exhibited O–H stretching at 3200–3500 cm−1, a shoulder peak at around 2900 cm−1 associated with –CH2, the peaks at 1407 cm−1 and 1616 cm−1 related to carboxylate symmetric and asymmetric stretching vibration, respectively. The HA–Gd2O3 nanoprobe also exhibited the same typical peaks as pure HA, which demonstrated the presence of HA in the prepared nanoprobe (Fig. 1b).29,30 As shown in Fig. S3,† the typical 222 peak of Gd2O3 could be seen in the XRD pattern of the nanoprobe.31 To further investigate the oxidation state of Gd, XPS analysis was performed. As shown in Fig. S4,† Gd 4d spectrum was deconvoluted into two components, which was assigned to Gd 4d3/2 and Gd 4d5/2 in Gd2O3,32,33 respectively.
Stability measurements
The stability of the nanoprobe was estimated by DSL measurement, the result showed that the hydrodynamic diameter of HA–Gd2O3 dispersed in normal saline for 30 days had no notable difference to that of the nanoparticles stored for 1 day (Fig. S5†). The above experimental results indicated that the nanoprobe possessed good colloidal stability in saline solution, which could be used for further study in biological applications.T1 relaxivity and T1-weighted MR imaging in vitro
The T1 relaxivity characterization and in vitro MR imaging of the nanoprobe and Gd–DTPA were performed on a 0.5 T MR scanner. The r1 value of the contrast agents were calculated through the measurement of T1 value as a function of Gd3+ concentration. As shown in Fig. 2a, the HA–Gd2O3 nanoprobe showed a high r1 value of 14.95 mM−1 s−1, which was almost three times higher than that of Gd–DTPA (5.02 mM−1 s−1). In addition, compared to Gd–DTPA, HA–Gd2O3 shows significantly brighter T1-weighted MR images even with much lower concentrations of Gd3+ (Fig. 2b). | ||
| Fig. 2 (a) r1 relaxivity curves of HA–Gd2O3 nanoprobe; (b) T1 MR imaging in vitro of Gd–DTPA and HA–Gd2O3 nanoprobe at various Gd concentrations. | ||
The above results clearly indicated the proposed HA–Gd2O3 nanoprobe possesses a much higher sensitivity than the commercial Gd–DTPA agent. The results could be explained as follows: (1) compared to the small Gd3+ chelates with low relaxivity derived from rapid tumbling,12 the HA–Gd2O3 nanoprobe with a nanoscale size had a much slower tumbling, which resulted in a more efficient relaxation mechanism of the bound water;12 (2) HA could control the growth of Gd2O3 with a small size and increase the ratio of surface Gd3+ to inner Gd3+ ions in the crystal lattice, enabling Gd3+ interactions with water molecules;15,16 (3) hydrogen bond formation between water molecules and adjacent carboxyl and N-acetyl of HA leads to a unique water retention capacity of the polymer,28 and the increased local water density could improve the water exchange rate,26,27 leading to a high T1 relaxivity. The synergistic effects ensured the high r1 value of the HA–Gd2O3 nanoprobe.
In vitro cytotoxicity assay
In vitro cytotoxicity of the HA–Gd2O3 nanoprobe was investigated before the biological application. MTT assay was carried out to assess the HeLa cell viability after exposure to different concentrations of the HA–Gd2O3 nanoprobe for 24 h (Fig. 3a). The nanoprobe showed negligible cytotoxicity even when the concentration was as high as 1 mg mL−1, which was much higher than that in most of the previous reports of Gd-based nanoprobes.3 The cell uptake assay showed a small part of the nanoprobe was internalized by HeLa cells (Fig. S6†). Therefore, these results demonstrated that the HA–Gd2O3 nanoprobe possessed negligible cytotoxicity and an excellent biocompatibility. | ||
| Fig. 3 (a) MTT assay of HeLa cell viability after treatment with various concentrations of the HA–Gd2O3 nanoprobe for 24 h. (b) Body weight change of the test and control mice groups over a month. | ||
Toxicity assay in vivo
A biodistribution study involving body weight monitoring and histological changes of the major organs was carried out to further investigate the acute and long-term toxicity of the HA–Gd2O3 nanoprobe in vivo. The biodistribution of the HA–Gd2O3 nanoprobe in different organs was determined. The results indicated that the nanoprobe was mainly accumulated in the spleen and liver, a few nanoprobes were found in the kidney, and the nanoprobe was gradually metabolized over time (Fig. S7†). According to the previous study, the Gd2O3 nanoprobe probably decomposed in the lysosomes where the pH value is about 4.5 after endocytosis by the cells, and excreted out from the body gradually.34 As shown in Fig. 3b, there was no significant difference between the test and control group. In addition, compared with the control group, the test group also showed normal physical behavior over the experiment. To estimate whether the nanoprobe could cause any histopathological damages, the major organs (heat, liver, spleen, kidney and adrenal gland) of the mice were extracted at different time points post-injection of the nanoprobe and analyzed by H&E staining. The results, as shown in Fig. S8,† displayed no obvious lesions in the test group and their histomorphology showed no difference compared to the control group.Furthermore, blood biochemical analysis was also performed to assess the potential toxicity in mice at different time points post-injection of the nanoprobe. Several indicators were analyzed to reflect the hepatic and kidney function. As shown in Fig. 4, liver function indicators (TP, ALB, GLB, ALT, GGT and ASL) and kidney function indicators (UREA and CREA) of the test group were all measured and found to be normal. The results indicated that the nanoprobe had no notable toxicity to hepatic and kidney function. In view of the toxicity assay above, the HA–Gd2O3 nanoprobe exhibited high biocompatibility in vivo and could be used for further MR imaging in vivo.
 | ||
| Fig. 4 Blood biochemistry assessment of the mice after treatment with and without HA–Gd2O3 nanoprobe (200 μL, 0.025 mmol Gd per kg) at 1 and 7 days (n = 3). | ||
MR imaging in vivo
Encouraged by the excellent biocompatibility in vitro and in vivo and a high r1 value, MR imaging of mice using the proposed nanoprobe was also investigated. As shown in Fig. 5a, after injection of the HA–Gd2O3 nanoprobe (200 μL, 0.025 mmol kg−1) at a quarter of the dose in clinical usage, MR images showed a rapid vessel signal enhancement, meanwhile the major organs including spleen, liver and kidney became brighter with excellent contrast resulting 5 min later. At 1-h post injection, the vessels showed the strongest MR signal, the long blood circulation indicated that the nanoprobe could be used as a good contrast agent for blood pool imaging, and the signal in the liver and spleen retained its strong intensity, while the MR image of the kidney became darker. Like most reported nanoprobes, the HA–Gd2O3 nanoprobe was mainly accumulated in the liver and spleen containing RES system, which revealed that the nanoprobe was in the main finally metabolized in the liver. At 24-h post injection, the MR signal in the whole body became much weaker, which indicated the probe was mostly metabolized.Organ-targeted MR imaging plays a crucial role in the diagnosis of various organ morphology-associated diseases.27,35 However, the fabrication of other targeted organ contrast agents besides the liver and kidney was more difficult than that of the tumor targeted ones due to the lack of specific interaction between the ligand and receptor. Fortunately, the adrenal gland-targeted ability was found in our developed nanoprobe. As shown in Fig. 5a, the adrenal gland also showed a remarkable signal enhancement at 5-min post injection of nanoprobe, and the strong signal did not decrease until after 6 h (Fig. S9†). That means the HA–Gd2O3 nanoprobe possesses a specific ability for adrenal gland MR imaging. The mechanism of this phenomenon was not clear, and other HA modified nanoparticles did not show adrenal gland specific imaging. The targeting ability may be associate with the physical and chemical properties of the HA–Gd2O3 nanoprobe and some unclear specific ingestion of the adrenal gland, which needs further investigation in terms of the molecular biology involved. MR imaging of mice using Gd–DTPA was also performed for comparison. As shown in Fig. 5b, at 5-min post injection of Gd–DTPA (200 μL, 0.025 mmol Gd per kg), MR images showed a rapid signal enhancement in the liver and kidney after injection and a fast excretion through urine subsequently. Even when the mice were treated with a clinically-used dose of Gd–DTPA (200 μL, 0.1 mmol Gd per kg), there was no noticeable improvement for the blood circulation time. At 1-h post injection, there was a significant decline in the signal of the whole body, which indicated that most of the Gd–DTPA had been cleared from the body. In addition, there was no significant signal enhancement in the adrenal gland with both low and high dosages of Gd–DTPA. The above results further evidence that the HA–Gd2O3 nanoprobe possesses high sensitivity and organ-targeted ability.
Conclusion
To summarize, we used HA as a template to synthesise a high sensitive HA–Gd2O3 nanoprobe with good biocompatibility by a facile one-pot reaction at room temperature. Based on the SBM theory, HA facilitated an increase in the number and percentage of effective Gd3+ in one particle, and the extension of the rotational tumbling time (τR) of inner sphere water molecules. The synergistic effects efficiently improved the r1 value of the HA–Gd2O3 nanoprobe. In addition, HA as an inherently human molecule ensured excellent biocompatibility of the HA–Gd2O3 nanoprobe. As a result, the HA–Gd2O3 nanoprobe has higher r1 values, long blood circulation times and excellent biocompatibility in vitro and in vivo. In addition, the proposed nanoprobe shows a significant organ targeting ability, and could be used for adrenal gland MR imaging in vivo, showing great potential application in evaluating the morphology of adrenal gland or other adrenal gland diseases. More importantly, the proposed method provides a new strategy for the design of high sensitive and biocompatible MR nanoprobes towards clinical applications.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21405112, 21435001), China Postdoctoral Science Foundation (Grants 2014M550146).Notes and references
- S. Aime, M. Botta, M. Fasano and E. Terreno, Chem. Soc. Rev., 1998, 27, 19–29 RSC.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- Y. Liu and N. Zhang, Biomaterials, 2012, 33, 5363–5375 CrossRef CAS PubMed.
- K. N. Raymond and V. C. Pierre, Bioconjugate Chem., 2005, 16, 3–8 CrossRef CAS PubMed.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372–390 RSC.
- D. E. Lee, H. Koo, I. C. Sun, J. H. Ryu, K. Kim and I. C. Kwon, Chem. Soc. Rev., 2012, 41, 2656–2672 RSC.
- P. P. Adiseshaiah, J. B. Hall and S. E. McNeil, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 99–112 CrossRef CAS PubMed.
- A. M. Nystrom and B. Fadeel, J. Controlled Release, 2012, 161, 403–408 CrossRef PubMed.
- A. Dhawan and V. Sharma, Anal. Bioanal. Chem., 2010, 398, 589–605 CrossRef CAS PubMed.
- J. Ai, E. Biazar, M. Jafarpour, M. Montazeri, A. Majdi, S. Aminifard, M. Zafari, H. R. Akbari and H. G. Rad, Int. J. Nanomed., 2011, 6, 1117–1127 CAS.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS PubMed.
- P. Caravan, Chem. Soc. Rev., 2006, 35, 512–523 RSC.
- E. L. Que and C. J. Chang, Chem. Soc. Rev., 2010, 39, 51–60 RSC.
- G.-L. Davies, I. Kramberger and J. J. Davis, Chem. Commun., 2013, 49, 9704–9721 RSC.
- F. Chen, W. B. Bu, S. J. Zhang, X. H. Liu, J. N. Liu, H. Y. Xing, Q. F. Xiao, L. P. Zhou, W. J. Peng, L. Z. Wang and J. L. Shi, Adv. Funct. Mater., 2011, 21, 4285–4294 CrossRef CAS.
- J. Y. Park, M. J. Baek, E. S. Choi, S. Woo, J. H. Kim, T. J. Kim, J. C. Jung, K. S. Chae, Y. Chang and G. H. Lee, ACS Nano, 2009, 3, 3663–3669 CrossRef CAS PubMed.
- H. Otsuka, Y. Nagasaki and K. Kataoka, Adv. Drug Delivery Rev., 2012, 64, 246–255 CrossRef.
- N. Ma, A. F. Marshall and J. H. Rao, J. Am. Chem. Soc., 2010, 132, 6884–6885 CrossRef CAS PubMed.
- J. P. Xie, Y. G. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- C. L. Guo and J. Irudayaraj, Anal. Chem., 2011, 83, 2883–2889 CrossRef CAS PubMed.
- P. Wu, T. Zhao, Y. Tian, L. Wu and X. Hou, Chem.–Eur. J., 2013, 19, 7473–7479 CrossRef CAS PubMed.
- B. Zhang, H. Jin, Y. Li, B. Chen, S. Liu and D. Shi, J. Mater. Chem., 2012, 22, 14494–14501 RSC.
- S.-K. Sun, L.-X. Dong, Y. Cao, H.-R. Sun and X.-P. Yan, Anal. Chem., 2013, 85, 8436–8441 CrossRef CAS PubMed.
- C. I. Richards, S. Choi, J. C. Hsiang, Y. Antoku, T. Vosch, A. Bongiorno, Y. L. Tzeng and R. M. Dickson, J. Am. Chem. Soc., 2008, 130, 5038–5039 CrossRef CAS PubMed.
- B. P. Toole, Nat. Rev. Cancer, 2004, 4, 528–539 CrossRef CAS PubMed.
- H.-J. Cho, H. Y. Yoon, H. Koo, S.-H. Ko, J.-S. Shim, J.-H. Cho, J. H. Park, K. Kim, I. C. Kwon and D.-D. Kim, J. Controlled Release, 2012, 162, 111–118 CrossRef CAS PubMed.
- M. Moon, R. Thomas, S.-u. Heo, M.-S. Park, W. Bae, S. Heo, N. Yim and Y. Jeong, Mol. Imag. Biol., 2015, 1–7 Search PubMed.
- T. V. Anilkumar, J. Muhamed, A. Jose, A. Jyothi, P. V. Mohanan and L. K. Krishnan, Biologicals, 2011, 39, 81–88 CrossRef CAS PubMed.
- E. K. Lim, H. O. Kim, E. Jang, J. Park, K. Lee, J. S. Suh, Y. M. Huh and S. Haam, Biomaterials, 2011, 32, 7941–7950 CrossRef CAS PubMed.
- M. Yu, S. Jambhrunkar, P. Thorn, J. Chen, W. Gu and C. Yu, Nanoscale, 2013, 5, 178–183 RSC.
- F. Söderlind, H. Pedersen, R. M. Petoral Jr, P.-O. Käll and K. Uvdal, J. Colloid Interface Sci., 2005, 288, 140–148 CrossRef PubMed.
- D. Raiser and J. P. Deville, J. Electron Spectrosc. Relat. Phenom., 1991, 57, 91–97 CrossRef CAS.
- A. T. M. Anishur Rahman, K. Vasilev and P. Majewski, J. Colloid Interface Sci., 2011, 354, 592–596 CrossRef CAS PubMed.
- Y. Yang, Y. Sun, Y. Liu, J. Peng, Y. Wu, Y. Zhang, W. Feng and F. Li, Biomaterials, 2013, 34, 508–515 CrossRef CAS PubMed.
- C. J. Sun, H. Yang, Y. Yuan, X. Tian, L. M. Wang, Y. Guo, L. Xu, J. L. Lei, N. Gao, G. J. Anderson, X. J. Liang, C. Y. Chen, Y. L. Zhao and G. J. Nie, J. Am. Chem. Soc., 2011, 133, 8617–8624 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of optimization of synthetic conditions, XRD and XPS characterization, HE analysis, distribution in major organs in vivo of HA–Gd2O3. See DOI: 10.1039/c5ra12704a |
| This journal is © The Royal Society of Chemistry 2015 |


