Overexpression of hgc1 increases the production and diversity of hygrocins in Streptomyces sp. LZ35†
Shanren Li‡
,
Chunhua Lu‡,
Jinhuan Ou,
Jingjing Deng and
Yuemao Shen*
Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Shandong University, Jinan 250012, P. R. China. E-mail: yshen@sdu.edu.cn; Tel: +86-531-88382108
First published on 25th September 2015
Abstract
Manipulation of pathway regulation is an efficient strategy to increase the secondary metabolite production. The production of hygrocins in Streptomyces sp. LZ35 was previously increased by overexpression of the hgc1, a LAL-family pathway-specific activator gene. In this study, we have further characterized the products of the hgc1-overexpressed mutant and three new hygrocins were isolated with the aid of chromophore-guided fractionation. The structures of hygrocins H–J (1–3) were determined by the analysis of the 1D and 2D NMR spectroscopic and high-resolution mass spectrometry data. Hygrocin H (1) was determined as 2,19-dehydrated-hygrocin C; hygrocin I (2) and J (3) were shown to be 13,14-seco-hygrocin H and 13,14-seco-2,19-dehydrated hygrocin F, respectively. Hygrocin H showed toxicity to human tumor MDA-MB-231, PC3 and HeLa cell lines (IC50 = 2.4, 1.7, and 0.8 μM, respectively), while hygrocins I and J were inactive at 50 μM against all the tested cell lines.
Introduction
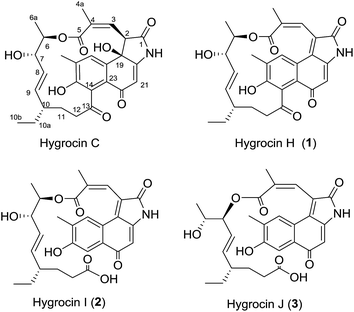
Ansamycins are an important family of natural products that exhibit a range of biological activities, including the Hsp90 inhibitor geldanamycin,1 the RNA polymerase inhibitor rifamycin,2 and the antiproliferative maytansinoids.3 The hygrocins, structurally diverse naphthalenic ansamycins, were first isolated from Streptomyces hygroscopicus in 2005, have been shown to possess anti-bacterial and anti-cancer activity.4,5 Additionally, the hygrocins C–G were recently isolated from the gdmAI-disrupted Streptomyces sp. LZ35.5 The intriguing structures and excellent bioactivity of hygrocins have encouraged us to search for more congeners.In many species of Streptomyces, the secondary metabolites biosynthetic genes are clustered on the chromosome or plasmids.6 The biosynthesis of each type of antibiotics is usually controlled by regulatory proteins, especially by transcriptional activators. Overexpression of pathway-specific activator genes have been reported to lead to increased production of the corresponding antibiotics.7 Recently, the biosynthesis of hygrocins has been studied, and found that hgc1 is a specific LAL-type activator in hygrocin biosynthesis.8 To increase the production of hygrocins for facilitating isolation, a strain SR101OEhgc1 was constructed by constitutive overexpression (OE) of hgc1.8 By metabolic profiling using HPLC/diode array detection (DAD), we found that the hygrocin congeners produced by SR101OEhgc1 are more abundant than expected. Further fractionation and detailed isolation by a combination of various column chromatographic methods with the aid of DAD of naphthoquinone or naphthohydroquinone chromophore led to the identification of three new hygrocin congeners, namely hygrocins H–J (1–3) (Fig. 1). Herein, we report the isolation, structure elucidation and cytotoxicity evaluation of the three new hygrocin analogues, which illuminates the flexibility and diversity of hygrocin biosynthesis.
Results and discussion
The molecular formula C28H29NO7 of 1 was established by HRESIMS (m/z 492.2030 [M + H]+). The 1H NMR spectrum (Table 1) displayed the presence of four methyl groups (δH 0.74, t; 0.95, d; 2.28, br s and 2.25, s), two oxymethine protons (δH 4.89, m and 3.87, q) and five aromatic and/or double-bond protons (δH 7.67, s; 6.96, s; 5.93, s; 5.38, dd and 4.61, dd). The 13C NMR and HMQC data of 1 revealed the presence of four methyl carbons (δC 13.4, 12.5, 17.0 and 20.9), three methylene carbons (δC 26.5, 31.8 and 39.5), two oxymethine carbons (δC 71.2 and 74.6), twelve aromatic or olefinic carbons (δC 106.0, 126.3, 128.4, 128.5, 128.4, 131.2, 132.8, 134.1, 137.6, 137.2, 156.1 and 155.8), and two carboxyls (δC 167.3 and 172.4), one α,β-unsaturated keto carbon (δC 186.8) and one keto carbon at δC 211.9 (ESI Fig. S5–S7†). The 1H and 13C NMR spectral data of 1 were similar to those of hygrocin C.5 The major difference between hygrocin C and 1 are the changes of chemical shifts at C-19 (δC 72.6 d in hygrocin C, δC 131.4 s in 1), and C-2 (δC 53.5 d, δH 4.67 in hygrocin C, and δC 126.3 s in 1), which indicated that the carbon–carbon double bond was formed between C-19 and C-2 due to dehydration. Additionally, the structure of 1 was further confirmed by HMBC and 1H–1H COSY correlations (Fig. 2, ESI Fig. S8–S9†). The relative configurations of 1 was established similar to those of hygrocin C by the coupling constants (J = 15.5 Hz) between H-8 and H-9 and the relative downfield shift of the allylic methyl group C-4a (δC 20.9).| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| 1H J = Hz | 13C | 1H J = Hz | 13C | 1H J = Hz | 13C | |
| a Those signals were estimated from HMBC correlations. | ||||||
| 1 | 172.4a (s) | 171.4 (s) | 171.5 (s) | |||
| 2 | Not observed | 126.6 (s) | 126.6 (s) | |||
| 3 | 6.96 br s | 128.4 (d) | 7.54 s | 129.8 (d) | 7.53 d (1.2) | 129.6 (d) |
| 4 | 137.6 (d) | 137.5 (s) | 137.5 (s) | |||
| 4a | 2.25 d (1.3) | 20.9 (q) | 1.92 br s | 16.3 (q) | 1.92 d (1.4) | 16.2 (q) |
| 5 | 167.3 (s) | 167.9 (s) | 167.8 (s) | |||
| 6 | 4.89 (Overlapped in D2O) | 74.6 (d) | 5.08 dq (6.1, 5.2) | 75.3 (d) | 3.98 dq (6.4, 6.4) | 69.7 (d) |
| 6a | 0.94 d (6.4) | 13.4 (q) | 1.35 d (6.1) | 16.7 (q) | 1.26 d (6.4) | 19.5 (q) |
| 7 | 3.87 q (1.7) | 71.2 (d) | 4.20 t (5.2) | 75.4 (d) | 5.25 t (6.4) | 81.2 (d) |
| 8 | 4.61 dd (15.5, 9.1) | 128.5 (d) | 5.52 dd (15.5, 8.3) | 131.3 (d) | 5.57 m | 127.6 (d) |
| 9 | 5.38 dd (15.5, 1.5) | 137.2 (d) | 5.57 dd (15.5, 6.3) | 138.2 (d) | 5.58 m | 141.2 (d) |
| 10 | 1.59 m | 43.7 (d) | 1.95 m | 45.5 (d) | 1.98 m | 45.7 (d) |
| 10a | 1.53 m, 0.99 m | 26.5 (t) | 1.46 m, 1.31 m | 29.1 (t) | 1.56 m, 1.35 m | 28.9 (t) |
| 10b | 0.74 t (7.3) | 12.5 (q) | 0.90 t (7.2) | 12.2 (q) | 0.89 t (7.3) | 12.2 (q) |
| 11 | 1.34 m, 1.20 m | 31.8 (t) | 1.75 m, 1.46 m | 31.1 (t) | 1.79 m, 1.56 m | 31.2 (t) |
| 12 | 2.82 m | 39.5a (t) | 2.28 m | 33.1 (t) | 2.33 m | 33.5 (t) |
| 13 | 211.9a (s) | 177.9 (s) | 178.5a (s) | |||
| 14 | Not observed | 7.44 s | 113.7 (d) | 7.41 s | 113.8 (d) | |
| 15 | 156.1a (s) | 159.7 (s) | 159.7 (s) | |||
| 16 | 132.8a (s) | 131.8 (s) | 131.7 (s) | |||
| 16a | 2.28 br s | 17.0 (q) | 2.24 s | 16.3 (q) | 2.20 s | 16.6 (q) |
| 17 | 7.67 s | 131.2 (d) | 7.43 s | 131.5 (d) | 7.38 s | 131.4 (d) |
| 18 | 131.2 (s) | 122.6 (s) | 122.6 (s) | |||
| 19 | 134.1 (s) | 137.3 (s) | 137.2 (s) | |||
| 20 | 155.8a (s) | 153.9 (s) | 153.8 (s) | |||
| 21 | 5.93 s | 106.0 (d) | 5.89 s | 106.1 (d) | 5.87 s | 106.2 (d) |
| 22 | 186.8 (s) | 186.3 (s) | 186.3 (s) | |||
| 23 | 129.4 (s) | 131.7 (s) | 131.7 (s) | |||
Hygrocins I (2) and J (3) were obtained both as red powder with [α]20D − 18 (c 0.40, CH3OH) and [α]25D − 30 (c 0.42, CH3OH), and HRESIMS data indicated that 2 and 3 have the same molecular formula of C28H31NO8 (m/z 510.2045 [M + H]+). Detailed comparison of the NMR data (Table 1) of 2 and 1 revealed the apparent differences. The 1H NMR spectra revealed the presence of an aromatic proton at δH 7.44 (H-14). The changes of chemical shift at C-14 (δC 128.4 s in 1, δC 113.7 d in 2) and C-13 (δC 211.9 s in 1; δC 177.9 s in 2) indicated the breakage of C-13/C-14 bond, which was further supported by the HMBC correlations from H-14 to C-16, C-18 and C-22 (Fig. 2). Thus, compound 2 was determined to be 13,14-seco-hygrocin H.
Hygrocin J (3), the 1D and 2D-NMR data revealed that this metabolite represents a homologue of 2, but differs in the ester linkage of side chain (Fig. 1 and 2). The downfield shift of H-7 (δH 5.25) and upfield shift of H-6 (δH 3.98) indicated the formation of a C-7/5 ester linkage instead of a C-6/5 in 3 (Table 1), which is similar to the difference between hygrocins E and F.5 Therefore, compound 3 was determined to be 13,14-seco-2,19-dehydrated hygrocin F.
Hygrocins H-J were tested for their cytotoxicities against human tumor MDA-MB-231, PC3 and HeLa cell lines. Hygrocin H was found to be toxic to MDA-MB-231, PC3 and HeLa cell lines with IC50 of 2.4, 1.7, and 0.8 μM, respectively, while hygrocins I and J were inactive at the concentration of 50 μM, which suggested that the ansa ring was important for the biological activity.
The two seco-derivatives hygrocins I and J (2 and 3, respectively) may derive from a spontaneous reversed-Claisen reaction, in which deprotonation of phenolic oxygen and protonation of the alpha carbon leads to a highly conjugated, resonance-stabilized tautomer, which is then vulnerable to hydrolysis and ring opening (Scheme 1). Basic or acidic conditions might promote this reaction. We speculate that the high degree of conjugation in the tricyclic system of the hygrocins allows the formation of the tautomer and the otherwise difficult cleavage of the C–C bond between the phenolic moiety and macrocycle.
The intermediates of ansamycin have been observed in mutated pathways of rifamycin.9 And the recently reported the seco-variants of ansamycins divergolides M and N are shunt products of the biosynthetic pathway and seco-divergolide L maybe formed by decarboxylation after spontaneous hydrolysis of the macrolide.10 Whereas, seco-hygrocin congeners (2 and 3) are different in that the C–C bond at the aromatic ring is cleaved. Therefore, the seco-variants 2 and 3, isolated from the strain SR101OEhgc1 in this study, represented the novel examples of natural seco-ansamycins.
Experimental section
General experimental procedures
HRESIMS were carried out on an LTQ-Orbitrap XL. NMR spectra were measured on Bruker DRX-600 MHz NMR spectrometer (Bruker Daltonics Inc., Billerica, Massachusetts) with tetramethylsilane (TMS) as an internal standard. Reversed-phase (RP) C18 silica gel for column chromatography (CC) was obtained from Merck (Darmstadt, Germany) and Sephadex LH-20 from GE Amersham Biosciences (Piscataway, New Jersey). Silica gel (200–300 mesh) for CC and silica gel GF254 for TLC were purchased from Qingdao Marine Chemical Ltd (Qingdao, China). High-performance liquid chromatography (HPLC) was performed using ZORBAX XDB-C18 (5 μm). Semi preparative column (9.4 × 250 mm). All solvents used were of analytical grade. Compounds were visualized under UV light and by spraying with H2SO4/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) followed by heating.
9, v/v) followed by heating.
Strain and fermentation
Strain SR101OEhgc1 was constructed by our previously work.8 The strain was cultured and fermented for 11 d in Petri dishes laid with ca. 20 mL ISP3 medium (1.5% agar, 2% oatmeal, 0.1% trace element solution, pH 7.2) with a total volume of 10 litres at 28 °C.Extraction and isolation
To extract the metabolites, the culture of SR101OEhgc1 was diced and extracted three times overnight with EtOAc/MeOH/AcOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v) at room temperature and partitioned between EtOAc and doubly-distilled water until the EtOAc layer was colorless. The EtOAc soluble fraction was dried with sodium sulfate (anhydrous) and the solvent was removed under vacuum to afford the EtOAc extract. The EtOAc extract was sequentially solvent-partitioned into petroleum ether and 95% aqueous methanol soluble extracts. The methanol extract (2.0 g) was subjected to CC over Sephadex LH-20 (140 g) eluted with acetone to obtain 8 fractions, i.e. Fr. 1–8. HPLC analysis indicated that Fr. 6 and Fr. 7 contained compounds with differential absorption (ESI Fig. S1†). Fr. 6 (396 mg) was further subjected to MPLC over RP-18 silica gel (40 g), subfractions were obtained from the elutions of 30%-100 mL, 40%-100 mL, 50%-200 mL, 70%-100 mL and 100%-100 mL MeOH in water, respectively. While 16 mL were collected for each subfraction, 1–6 were obtained from 30%, 7–12 from 40%, 13–24 from 50%, 25–31 from 70% and 32–35 from 100% MeOH. According to TLC results, the subfractions 1–12, 13–15, 16–20, 21–31 and 32–35 were combined and marked as Fr. 6a, Fr. 6b, Fr. 6c, Fr. 6d and Fr. 6e, respectively. HPLC analysis of the constituents of Fr. 6a–e indicated that compounds with differential absorption existed in Fr. 6c. Fr. 6c (20 mg) was finally purified by semi-preparative reversed-phase HPLC (Agilent 1260 instrument; ZORBAX Eclipse XDB-C18 5 μm, column ID: 9.4 × 250 mm, flow rate: 4 mL min−1, elution: CH3CN/H2O (35–65, v/v), UV detections at 274 nm) to afford 1 (tR 7.7 min, 6 mg) (ESI Fig. S2†). Fr. 7 (478 mg) was further subjected to MPLC over RP-18 silica gel 40 g, 13 subfractions were obtained and marked as Fr. 7a–7M. Fr. 7d (50 mg) was subjected to Sephadex LH-20 (80 g) eluted with acetone to obtain Fr. 7d1 (ESI Fig. S3†). Fr. 7d1 (12 mg) was finally purified by semi preparative reversed-phase HPLC (Agilent 1260 instrument; ZORBAX Eclipse XDB-C18 5 μm, column ID: 9.4 × 250 mm, flow rate: 4 mL min−1, elution: CH3CN/H2O (40–60, v/v), UV detections at 274 nm) to afford 3 (tR 8.5 min, 3 mg) and 2 (tR 9.2 min, 3 mg) (ESI Fig. S4†).
5, v/v/v) at room temperature and partitioned between EtOAc and doubly-distilled water until the EtOAc layer was colorless. The EtOAc soluble fraction was dried with sodium sulfate (anhydrous) and the solvent was removed under vacuum to afford the EtOAc extract. The EtOAc extract was sequentially solvent-partitioned into petroleum ether and 95% aqueous methanol soluble extracts. The methanol extract (2.0 g) was subjected to CC over Sephadex LH-20 (140 g) eluted with acetone to obtain 8 fractions, i.e. Fr. 1–8. HPLC analysis indicated that Fr. 6 and Fr. 7 contained compounds with differential absorption (ESI Fig. S1†). Fr. 6 (396 mg) was further subjected to MPLC over RP-18 silica gel (40 g), subfractions were obtained from the elutions of 30%-100 mL, 40%-100 mL, 50%-200 mL, 70%-100 mL and 100%-100 mL MeOH in water, respectively. While 16 mL were collected for each subfraction, 1–6 were obtained from 30%, 7–12 from 40%, 13–24 from 50%, 25–31 from 70% and 32–35 from 100% MeOH. According to TLC results, the subfractions 1–12, 13–15, 16–20, 21–31 and 32–35 were combined and marked as Fr. 6a, Fr. 6b, Fr. 6c, Fr. 6d and Fr. 6e, respectively. HPLC analysis of the constituents of Fr. 6a–e indicated that compounds with differential absorption existed in Fr. 6c. Fr. 6c (20 mg) was finally purified by semi-preparative reversed-phase HPLC (Agilent 1260 instrument; ZORBAX Eclipse XDB-C18 5 μm, column ID: 9.4 × 250 mm, flow rate: 4 mL min−1, elution: CH3CN/H2O (35–65, v/v), UV detections at 274 nm) to afford 1 (tR 7.7 min, 6 mg) (ESI Fig. S2†). Fr. 7 (478 mg) was further subjected to MPLC over RP-18 silica gel 40 g, 13 subfractions were obtained and marked as Fr. 7a–7M. Fr. 7d (50 mg) was subjected to Sephadex LH-20 (80 g) eluted with acetone to obtain Fr. 7d1 (ESI Fig. S3†). Fr. 7d1 (12 mg) was finally purified by semi preparative reversed-phase HPLC (Agilent 1260 instrument; ZORBAX Eclipse XDB-C18 5 μm, column ID: 9.4 × 250 mm, flow rate: 4 mL min−1, elution: CH3CN/H2O (40–60, v/v), UV detections at 274 nm) to afford 3 (tR 8.5 min, 3 mg) and 2 (tR 9.2 min, 3 mg) (ESI Fig. S4†).
Hygrocin H (1): yellow powder; [α]20D = +16.7 (c 0.35, CH3OH). UV(MeOH) λmax, 260, 295, 335, 380 nm. 1H and 13C NMR data, see Table 1. HRESIMS m/z 492.2030 [M + H]+ (calcd for C28H29NO7+, 492.2017).
Hygrocin I (2): red powder; [α]20D = −18 (c 0.40, MeOH); UV(MeOH) λmax, 220, 260, 285, 335, 380 nm. 1H and 13C NMR data, see Table 1. HRESIMS m/z 510.2045 [M + H]+ (calcd for C28H29NO8+, 510.2122).
Hygrocin J (3): red powder; [α]20D = −30 (c 0.42, MeOH); UV(MeOH) λmax, 220, 260, 285, 335, 380 nm. 1H and 13C NMR data, see Table 1. HRESIMS m/z 510.2045 [M + H]+ (calcd for C28H29NO8+, 510.2122).
Cytotoxicity test
The in vitro antiproliferative activities were assessed with a sulforhodamine B (SRB) assay.11 The test compounds and hygrocin C were dissolved in DMSO at 50 mM as a stock solution, respectively. Cells were seeded in triplicate in 96-well plates at a density of 5000 cells per well and incubated for 24 h in 0.1 mL of culture medium, leaving three wells without cell seeded as blank control. Then the medium in each well was exchanged with 0.1 mL of medium containing graded concentrations of compounds or same volume of DMSO and incubated for 24, 36, 48 or 72 h. Media were discarded and 10% trichloroacetic acid (TCA) was added to cell monolayers and stained for 1 h at 4 °C. TCA was removed by washed with distilled water for five times, after which 100 μL 4 mg mL−1 SRB (Sigma-Aldrich) was added and stained for 15 min at room temperature. After excessive dye being removed by washing five times with 1% acetic acid, 200 μL 10 mM Tris base solution was used to dissolve protein-bound dye, then measured the OD at 570 nm wavelength by a microplate reader (M-3350, Bio-Rad). Growth inhibition rates were calculated by the following equation and Prism 5 (GraphPad Software, Inc.) was used to determine IC50, which is defined as the concentration of compound that results in 50% growth inhibition at 72 h. Independent experiments were taken at least in duplicate to confirm the results.| Growth inhibitory rate = ODcontrol well − ODsample well/ODcontrol well − ODblank well. |
Conclusions
In summary, we have isolated and characterized three new hygrocin analogues from the LAL-family regulator gene hgc1-overexpressed strain SR101OEhgc1. In these compounds, hygrocin H showed toxicity to human cancer cell lines, but the seco-hygrocins I and J lost the activities with the inherent reactivity in ansamycin biosynthesis. This work not only demonstrates the overexpression of regulatory genes could be a useful strategy to increase antibiotic production, but also exhibits the unusual flexibility and diversity in ansamycin biosynthesis.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81373304, 81530091), the Fundamental Research Funds of Shandong University (2014JC027), Program for Changjiang Scholars and Innovative Research Team in University (IRT13028) and China Postdoctoral Science Foundation Funded Project (2014M561915).Notes and references
- Y. Fukuyo, C. R. Hunt and N. Horikoshi, Cancer Lett., 2010, 290, 24–35 CrossRef CAS PubMed.
- H. G. Floss and T. W. Yu, Chem. Rev., 2005, 105, 621–632 CrossRef CAS PubMed.
- J. M. Cassady, K. K. Chan, H. G. Floss and E. Leistner, Chem. Pharm. Bull., 2004, 52, 1–26 CrossRef CAS.
- P. Cai, F. Kong, M. E. Ruppen, G. Glasier and G. T. Carter, J. Nat. Prod., 2005, 68, 1736–1742 CrossRef CAS PubMed.
- C. Lu, Y. Li, J. Deng, S. Li, Y. Shen, H. Wang and Y. Shen, J. Nat. Prod., 2013, 76, 2175–2179 CrossRef CAS PubMed.
- G. Liu, K. F. Chater, G. Chandra, G. Niu and H. Tan, Microbiol. Mol. Biol. Rev., 2013, 77, 112–143 CrossRef CAS PubMed.
- Y. Chen, M. J. Smanski and B. Shen, Appl. Microbiol. Biotechnol., 2010, 86, 19–25 CrossRef CAS PubMed.
- S. Li, H. Wang, Y. Li, J. Deng, C. Lu, Y. Shen and Y. Shen, ChemBioChem, 2014, 15, 94–102 CrossRef CAS PubMed.
- T. W. Yu, Y. M. Shen, Y. Doi-Katayama, L. Tang, C. Park, B. S. Moore, C. R. Hutchinson and H. G. Floss, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 9051–9056 CrossRef CAS.
- L. Ding, J. Franke and C. Hertweck, Org. Biomol. Chem., 2015, 13, 1618–1623 CAS.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112–1116 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12623a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |

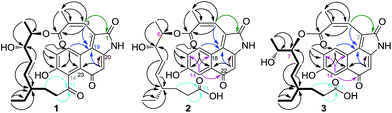
![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and HMBC (→) correlations for compounds 1–3.
) and HMBC (→) correlations for compounds 1–3.