Intramolecular triplet–triplet energy transfer enhanced triplet–triplet annihilation upconversion with a short-lived triplet state platinum(II) terpyridyl acetylide photosensitizer†
Shuai Yua,
Yi Zeng*a,
Jinping Chena,
Tianjun Yua,
Xiaohui Zhanga,
Guoqiang Yang*b and
Yi Li*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: zengyi@mail.ipc.ac.cn; yili@mail.ipc.ac.cn; Fax: +86-10-82543518; Tel: +86-10-82543518
bBeijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: gqyang@iccas.ac.cn
First published on 12th August 2015
Abstract
A model of a dendritic compound (Pt–DPA) with two Pt-complex photosensitizer chromophores and two 9,10-diphenylanthracene (DPA) acceptor groups covalently attached to the periphery and the core of the poly(aryl ether) dendrimer of generation 1 was prepared. A triplet–triplet annihilation upconversion (TTA-UC) system (Pt–DPA/DPA–OH) was constructed in deaerated DMF by combining Pt–DPA with a dissociative acceptor (DPA–OH). Although the lifetime of the triplet state of the Pt-complex is only 52 ns, the upconversion fluorescence from DPA (400–460 nm) in the Pt–DPA/DPA–OH system was observed with a quantum yield of 0.22% upon selective excitation of the Pt-complex with a 473 nm laser, which is due to the efficient intramolecular triplet–triplet energy transfer (ΦTTET > 0.81) from the Pt-complex photosensitizer to the DPA acceptor within Pt–DPA. The acceptor covalently linked with the photosensitizer acts as an energy-relay to transfer the harvested energy to the dissociative acceptor which further undergoes the TTA process. The efficient intramolecular triplet–triplet energy transfer process between the photosensitizer and the acceptor plays an important role in the TTA-UC system building with a short-lived triplet state photosensitizer, which facilitates the production of the triplet state of the acceptor, thus advancing the TTA-UC process. This work presents a new strategy for construction of efficient TTA-UC systems utilizing short-lived triplet state photosensitizers.
Introduction
Photon upconversion refers to a process involved in conversion of lower-energy photons to higher-energy ones. Many efforts have been devoted to develop techniques and new materials for photon upconversion, because of its potential applications in solar energy conversion and biologic sciences. Photon upconversion has been achieved in two-photon absorption organic dyes, rare earth element doped nanoparticles, inorganic crystals, triplet–triplet annihilation upconversion (TTA-UC) systems,1–7 etc. Among them, TTA-UC has attracted much attention because of its unique superiority over other conventional upconversion techniques. First, TTA upconversion only needs a non-coherent excitation source with low-power (down to mW cm−2) to initiate the photophysical process. Second, the excitation and emission wavelength of the TTA-UC can be readily adjusted by the selection of photosensitizer and acceptor.A generic TTA-UC system is composed of a triplet photosensitizer and a triplet energy acceptor. The photosensitizer harvests the excitation energy to generate its excited singlet state, which sequently converts to its triplet state through the intersystem crossing (ISC). Afterwards, a triplet state of the acceptor is formed via triplet–triplet energy transfer (TTET) from the photosensitizer to the acceptor. When two acceptor molecules in the triplet state diffuse and collide virtually within their lifetimes, a singlet state and a ground state of the acceptor are produced by the triplet–triplet annihilation (TTA). Consequently, an anti-Stokes delayed fluorescence can be obtained from the produced singlet state of the acceptor. The overall upconversion quantum efficiency of the TTA-UC system is determined by following factors: the efficiency of the intersystem crossing of the photosensitizer (ΦISC), the efficiency of TTET from the photosensitizer to the acceptor (ΦTTET), the efficiency of TTA of the acceptor (ΦTTA), and the florescence quantum yield of the acceptor (ΦF). In most cases, transition metal complexes are selected as the photosensitizer because their ISC efficiency is near unity caused by the heavy atom effect. Aromatic hydrocarbons possessing high fluorescence quantum yield, such as anthracene derivatives, are promising candidates of the acceptor because the large splitting of their singlet-triplet energy gaps, permits the relevant levels of the triplet state of photosensitizer to be sandwiched between, which facilitates the necessary thermodynamics.8 Therefore, the upconversion efficiency of the TTA-UC system with certain photosensitizer and acceptor is determined by the TTET and TTA processes.
The mechanism for TTET is usually described by Dexter electron–exchange interaction and it requires strong electronic orbital overlap between donor and acceptor.9 Therefore, a long-lived triplet state of photosensitizer is necessitated to increase the diffusion distance and likely encounter an acceptor molecule within its lifetime.10 To prolong the triplet lifetime of photosensitizers of transition metal complexes, methods have been employed extensively such as incorporation of selected organic chromophores into MLCT structures, which can evidently enhance the photophysical properties of exhibited by the latter.11,12 A coumarin-containing cyclometalated iridium(III) complex with a long-lived ligand-localized triplet excited state (3IL state, τ = 75.5 μs) was reported by Zhao's group,13 and an upconversion quantum yield of 21.3% was obtained in a TTA-UC system using this complex as the photosensitizer. In contrast, no upconversion emission could be observed when the photosensitizer was replaced by the model complex with a triplet lifetime less than 1 μs. Similar design has been adopted in their series works14–16 and enhanced upconversion performance was observed. TTA is a special case of energy transfer via electron exchange interactions, which is affected by the concentration and the lifetime of the triplet state of acceptor in the TTA-UC system. In a certain TTA-UC system, a high TTET efficiency is essential for a satisfying TTA efficiency, because the triplet state of acceptor is produced via the TTET process. To increase the TTET efficiency, a method of increasing concentration of the acceptor has been applied. An example reported by Felix N. Castellano et al. demonstrated that the TTET efficiency increased with increasing the concentration of the acceptor, consequently, resulting in enhanced TTA-UC efficiency.8 This method has been applied in many TTA-UC systems to promote the TTET process from the photosensitizer to the acceptor, consequently, advancing the overall TTA-UC performance.17–19 However, many conventional fluorophores exhibit high fluorescence efficiency in dilute solution but suffer concentration self-quenching.20,21 Therefore, the acceptor concentration in TTA-UC solution systems usually keeps quite low to avoid the self-quenching effect, and concentration optimization is needed for efficient TTET process in some cases.22,23
Our previous studies on dendrimers in mimicking light-harvesting systems demonstrate that TTET can occur efficiently from the peripheral chromophores to the core via a through-space mechanism because the folded dendritic scaffold can bring the periphery and core groups together.24–26 In the present work, the photosensitizer and the acceptor are introduced into a model of dendritic structure (Pt–DPA) and a TTA-UC system is constructed by combining Pt–DPA with a dissociative acceptor (DPA–OH). The acceptor covalent-linked with the photosensitizer acts as an energy-relay to transfer the harvested energy to the dissociative acceptor, which further undergoes the TTA process, thus accomplishing the TTA-UC process. The efficient intramolecular TTET process plays an important role in the TTA-UC system with a photosensitizer of short-lived triplet state, which provides a new strategy for construction of efficient TTA-UC systems.
Experimental
Materials
Reagents of analytical purity were used without further purification, unless otherwise noted. Solvents used for synthesis were dried and distilled before use. Spectrum pure acetonitrile (CH3CN) was used for absorption and emission spectral measurements of tris(4,4′-dimethyl-2,2′-bipyridine)ruthenium bis(hexafluorophosphate). DMF used for the TTA-UC measurements was dried with MgSO4 and distilled under a N2 atmosphere.Instrumentation
1H NMR (400 MHz) spectra were recorded on a Bruker Avance Π-400 spectrometer. IR spectra were run on a Varian Excalibur 3100 spectrometer. MALDI-TOF mass spectrometry was performed on a Bruker Daltonics MicroFlex spectrometer. Melting points were measured with a XT4A apparatus. UV/Vis absorption spectra were recorded on a Shimadzu UV-2550PC spectrometer. Steady-state emission spectra were performed on a Hitachi F-4500 spectrometer or a Princeton Instrument SPEC-10:400B/LN coupled to an Acton SP2500 spectrograph. The nanosecond transient absorption spectra were performed on an Edinburgh LP-920 pump-probe spectroscopic setup. Samples for phosphorescence and transient absorption experiments were deaerated by purged with nitrogen for 20 minutes prior to measurement. All photophysical measurements were performed at ambient temperature.TTA-UC measurements
The TTA-UC measurements were carried out in DMF at ambient temperature. A 473 nm laser (PicoQuant LDH-D-C-470 laser head with PDL 828 Sepia II driver system, CW mode) was used as the excitation source and an Ophir Nova II power meter with PD300-3W photodetector was used to measure photons absorbed by the TTA-UC system. The diameter of the laser spot was approximately 1 mm. The emission spectra of the TTA-UC system were recorded by a Princeton Instrument SPEC-10:400B/LN coupled to an Acton SP2500 spectrograph. Samples were deaerated by purging with argon for 20 minutes prior to measurements. The upconversion quantum yields (ΦUC) were determined with tris(4,4′-dimethyl-2,2′-bipyridine) ruthenium bis(hexafluorophosphate) (ΦP = 0.074 in deaerated CH3CN) as the standard. The upconversion quantum yields were calculated with eqn (1), where ΦUC, A, I and η represent the upconversion fluorescence quantum yield, absorbance, integrated luminescence intensity and the refractive index of the solvents, sam and std represent the sample and the standard, respectively. The equation is multiplied by factor 2 in order to make the maximum quantum yield to be unity.
 | (1) |
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A white solid (1.38 g, 81% yield) was obtained, Mp: 88–91 °C. 1H NMR (400 MHz, CDCl3), δ (ppm): 4.63 (s, 2H), 4.99 (s, 4H), 6.48 (m, 1H), 6.60 (d, J = 2.0 Hz, 2H), 7.28 (d, J = 8.4 Hz, 4H), 7.50 (d, J = 8.4 Hz, 4H).
1). A white solid (1.38 g, 81% yield) was obtained, Mp: 88–91 °C. 1H NMR (400 MHz, CDCl3), δ (ppm): 4.63 (s, 2H), 4.99 (s, 4H), 6.48 (m, 1H), 6.60 (d, J = 2.0 Hz, 2H), 7.28 (d, J = 8.4 Hz, 4H), 7.50 (d, J = 8.4 Hz, 4H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get a white solid (76% yield), Mp: 68–73 °C. 1H NMR (400 MHz, CDCl3), δ (ppm): 3.09 (s, 2H), 4.61 (s, 2H), 5.02 (s, 4H), 6.49 (m, 1H), 6.60 (d, J = 2.4 Hz, 2H), 7.36 (d, J = 8.0 Hz, 4H), 7.50 (d, J = 8.4 Hz, 4H).
1) to get a white solid (76% yield), Mp: 68–73 °C. 1H NMR (400 MHz, CDCl3), δ (ppm): 3.09 (s, 2H), 4.61 (s, 2H), 5.02 (s, 4H), 6.49 (m, 1H), 6.60 (d, J = 2.4 Hz, 2H), 7.36 (d, J = 8.0 Hz, 4H), 7.50 (d, J = 8.4 Hz, 4H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9 mL). The resulting mixture was thoroughly degassed through three freeze–pump–thaw cycles, and then stirred at 84 °C overnight. The reaction was quenched with water, and the organic layer was extracted with chloroform, dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. The obtained crude product was purified by column chromatography on silica gel (chloroform) to get a white solid (0.39 g, 45% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 4.77 (d, J = 6.0 Hz, 2H), 5.29 (s, 4H), 6.81 (m, 3H), 7.34 (m, 8H), 7.55 (m, 14H), 7.71 (m, 12H). MALDI-TOF MS m/z: calcd for C61H44O3 ([M]+) 824.3, found 824.0.
1, 9 mL). The resulting mixture was thoroughly degassed through three freeze–pump–thaw cycles, and then stirred at 84 °C overnight. The reaction was quenched with water, and the organic layer was extracted with chloroform, dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. The obtained crude product was purified by column chromatography on silica gel (chloroform) to get a white solid (0.39 g, 45% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 4.77 (d, J = 6.0 Hz, 2H), 5.29 (s, 4H), 6.81 (m, 3H), 7.34 (m, 8H), 7.55 (m, 14H), 7.71 (m, 12H). MALDI-TOF MS m/z: calcd for C61H44O3 ([M]+) 824.3, found 824.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to get a white solid (91% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 4.54 (s, 2H), 5.28 (s, 4H), 6.82 (m, 3H), 7.34 (m, 8H), 7.49 (m, 4H), 7.58 (m, 10H), 7.71 (m, 12H).
4) to get a white solid (91% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 4.54 (s, 2H), 5.28 (s, 4H), 6.82 (m, 3H), 7.34 (m, 8H), 7.49 (m, 4H), 7.58 (m, 10H), 7.71 (m, 12H).
Compound 3. Under nitrogen atmosphere, compound 2 (0.083 g, 0.23 mmol) and DPA–Br (0.2 g, 0.23 mmol) were dissolved with 10 mL dried tetrahydrofuran in a 50 mL three-neck round-bottom flask, and then NaH (150 mg, 6.25 mmol) was added carefully in several times. The resulting mixture was stirred and refluxed for 7 hours. The reaction was quenched with water, and the organic layer was extracted with dichloromethane, dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure. The obtained crude product was purified by column chromatography on silica gel (dichloromethane/petroleum ether = 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get a white solid (0.11 g, 40% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 3.05 (s, 2H), 4.58 (d, J = 12.8 Hz, 4H), 5.03 (s, 4H), 5.27 (s, 4H), 6.51 (m, 1H), 6.67 (d, J = 2.0 Hz, 2H), 6.81 (s, 3H), 7.33 (m, 16H), 7.49 (m, 16H), 7.71 (m, 10H). MALDI-TOF MS m/z: calcd for C86H62O5 ([M]+) 1174.5, found 1174.4. FT-IR: ν/cm−1 2108 (νC
1) to get a white solid (0.11 g, 40% yield). 1H NMR (400 MHz, CDCl3), δ (ppm): 3.05 (s, 2H), 4.58 (d, J = 12.8 Hz, 4H), 5.03 (s, 4H), 5.27 (s, 4H), 6.51 (m, 1H), 6.67 (d, J = 2.0 Hz, 2H), 6.81 (s, 3H), 7.33 (m, 16H), 7.49 (m, 16H), 7.71 (m, 10H). MALDI-TOF MS m/z: calcd for C86H62O5 ([M]+) 1174.5, found 1174.4. FT-IR: ν/cm−1 2108 (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C).
C).
Pt–DPA. To a 100 mL Schlenk flask, compound 3 (56 mg, 0.0477 mmol), compound 4 (59 mg, 0.1 mmol), and CuI were added, and then the flask was purged with nitrogen, charged with dried DMF and triethylamine (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mL). The resulting mixture was thoroughly degassed through three freeze–pump–thaw cycles, and then stirred at room temperature for 20 hours away from light. LiClO4 (70 mg, 0.44 mmol) was added and the mixture was stirred for another 2 hours at room temperature. 50 mL water was added and the resulting mixture was filtered under reduced pressure to obtain a red solid. The crude product was purified by washing with toluene and CH3OH for several times to obtain a reddish brown solid (0.10 g, 88% yield). 1H NMR (400 MHz, DMSO-d6), δ (ppm): 2.40 (s, 6H), 4.54 (d, J = 36.0 Hz, 4H), 5.30 (d, J = 30.4 Hz, 8H), 6.59 (d, J = 13.6 Hz, 3H), 6.83 (d, J = 24.0 Hz, 3H), 7.17 (m, 8H), 7.52 (m, 37H), 7.75 (m, 9H), 8.21 (s, 4H), 8.52 (br, 8H), 8.89 (s, 4H). MALDI-TOF MS m/z: calcd for C130H94ClN6O9Pt2 ([M–ClO4]+) 2307.6, found 2306.9. FT-IR: ν/cm−1 2114 (νC
1, 10 mL). The resulting mixture was thoroughly degassed through three freeze–pump–thaw cycles, and then stirred at room temperature for 20 hours away from light. LiClO4 (70 mg, 0.44 mmol) was added and the mixture was stirred for another 2 hours at room temperature. 50 mL water was added and the resulting mixture was filtered under reduced pressure to obtain a red solid. The crude product was purified by washing with toluene and CH3OH for several times to obtain a reddish brown solid (0.10 g, 88% yield). 1H NMR (400 MHz, DMSO-d6), δ (ppm): 2.40 (s, 6H), 4.54 (d, J = 36.0 Hz, 4H), 5.30 (d, J = 30.4 Hz, 8H), 6.59 (d, J = 13.6 Hz, 3H), 6.83 (d, J = 24.0 Hz, 3H), 7.17 (m, 8H), 7.52 (m, 37H), 7.75 (m, 9H), 8.21 (s, 4H), 8.52 (br, 8H), 8.89 (s, 4H). MALDI-TOF MS m/z: calcd for C130H94ClN6O9Pt2 ([M–ClO4]+) 2307.6, found 2306.9. FT-IR: ν/cm−1 2114 (νC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C).
C).
Pt–M. Similar procedure for the synthesis of Pt–DPA was adopted, except compound 2 (50 mg, 0.14 mmol) was used instead of compound 3. The obtained crude product was purified by washing with toluene and CH3OH for several times, and then a dark red solid was obtained (0.19 g, 85% yield). 1H NMR (400 MHz, DMSO-d6), δ (ppm): 2.40 (s, 6H), 4.37 (d, J = 5.6 Hz, 2H), 5.27 (s, 4H), 6.50 (s, 3H), 7.17 (d, J = 8.0 Hz, 4H), 7.24 (d, J = 8.0 Hz, 4H), 7.41 (d, J = 8.0 Hz, 4H), 7.73 (d, J = 8.0 Hz, 4H), 7.80 (t, J = 6.4 Hz, 4H), 8.27 (t, J = 8.0 Hz, 4H), 8.50 (d, J = 8.0 Hz, 4H), 8.59 (s, 4H), 8.95 (d, J = 5.2 Hz, 4H). ESI-HR MS m/z: calcd for C69H52N6O3Pt2 ([M–Cl2O8]2+) 701.1699, found 701.1678. FT-IR: ν/cm−1 2116 (νC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C).
C).
Results and discussion
Synthesis and characterization of the compounds
The synthetic routes and the structures of these compounds are shown in Scheme 1. The model compound DPA–OH was prepared by the Suzuki–Coupling reaction of (10-phenylanthracen-9-yl) boronic acid with compound 1. DPA–OH was bromized and then reacted with compound 2 through the Williamson reaction to give the alkynyl compound 3. Pt–DPA was obtained by the reaction of compound 3 with [Pt(4′-p-tolyl-trpy)Cl]Cl (4) in DMF in the presence of CuI and triethylamine at ambient temperature. The model compound of the photosensitizer Pt–M was prepared with a reaction similar to that of Pt–DPA by using compound 2 instead of compound 3. The compounds Pt–DPA, Pt–M and DPA–OH were characterized using 1H NMR spectra, mass spectrometry (MALDI-TOF or ESI-TOF), and IR spectra.Steady-state absorption and emission properties
The absorption spectra of Pt–DPA, Pt–M, DPA–OH and a mixture of Pt–M and DPA–OH (Pt–M/DPA–OH, 1/1, molar ratio) were measured in DMF, as shown in Fig. 1. Pt–M exhibits an intense absorption band below 370 nm assigned to the intraligand transitions of terpyridyl and acetylide ligands, and a broad absorption band in the region of 370–550 nm assigned to the MLCT transitions.28 DPA–OH displays clearly vibronic-structured absorption bands with maxima at 339, 356, 375 and 396 nm, which are attributed to the π–π* transition. Pt–DPA possesses basically identical absorption features with a simple mixture of Pt–M and DPA–OH, indicating there is no measurable interaction between the Pt-complex and the DPA chromophores intramolecularly in the ground state. The fact that the MLCT absorption of Pt–M extends to a wavelength longer than that of DPA–OH permits the selective excitation of the Pt-complex in Pt–DPA, Pt–M/DPA–OH, and the mixture of Pt–DPA and DPA–OH (Pt–DPA/DPA–OH).The emission spectra of Pt–M, Pt–DPA, and Pt–M/DPA–OH (1/1, molar ratio) at the same concentration of the photosensitizer were recorded in deaerated DMF upon excitation with a laser of 473 nm (Fig. 2). The weak emission of the 3MLCT transitions of the photosensitizer with maximum at 621 nm was detected for all three samples upon selective excitation of the photosensitizer, but the intensity of the emission is much weaker in Pt–DPA. The free-energy change involved in an electron transfer process from the triplet state of the photosensitizer to DPA is calculated to be positive, thus, the electron transfer can be excluded for the cause of the emission quenching in Pt–DPA. The triplet energy of the photosensitizer (2.48 eV) estimated from its emission spectrum is higher than that of DPA (1.77 eV).29 Therefore, the emission quenching in Pt–DPA can be ascribed to the exothermic TTET from the photosensitizer to the DPA acceptor, which is further strengthened by the transient absorption measurements. Because there is no visible emission quenching was detected in Pt–M/DPA–OH (1/1, molar ratio) with same concentrations of the photosensitizer and the acceptor, the occurrence of the TTET in Pt–DPA is intramolecular. Although there is an intramolecular TTET in Pt–DPA, neither phosphorescence nor fluorescence from DPA was observed at ambient temperature. The fluorescence of DPA–OH located at the higher energy region upon excitation with 350 nm light is also shown in Fig. 2 in comparison with the emission of the photosensitizer.
Triplet–triplet annihilation upconversion
A TTA-UC system was constructed in deaerated DMF by combining Pt–DPA and DPA–OH. Selective excitation of the photosensitizer of Pt-complex with a 473 nm laser in the Pt–DPA/DPA–OH system ([Pt–DPA] = 1.0 × 10−5 M, [DPA–OH] = 1.0 × 10−4 M) results in anti-Stokes florescence of DPA with characteristic maxima at 417 and 436 nm (Fig. 3a), which indicates the existence of upconversion process. The slight difference of the upconverted fluorescence from that of DPA–OH is caused by the inner filter effect owing to the relatively high concentration of DPA–OH in the TTA-UC system, which is similar to that reported in literatures.30,31 The excitation power dependence of the upconverted fluorescence intensity was investigated by gradually increasing the laser power from 3.64 to 9.54 mW. The upconversion fluorescence intensity increases with the laser power but in different manners in low and high power regions, respectively. To gain an insight into the correlation between the upconversion fluorescence intensity and the laser power, a double logarithm plot of the upconversion fluorescence intensity as a function of the incident light power is shown in Fig. 3b. A nearly quadratic (slope = 1.7) dependency of the upconverted fluorescence intensity on the excitation power can be observed in low power region (<6.06 mW), while a nearly linear (slope = 1.3) relationship exists when the excitation power is above 7.10 mW. The nonlinearly photophysical process observed in low power region suggests that the upconversion fluorescence derives from a TTA mechanism. With increasing the incident light power, the upconversion quantum yield tends to be saturated, tending towards a linear dependency. This finding is in accordance with typical TTA-UC examples.30,32,33 The upconversion quantum yield of the Pt–DPA/DPA–OH system was determined to be 0.22% (laser power = 9.54 mW) by using tris(4,4′-dimethyl-2,2′-bipyridine)ruthenium bis(hexafluorophosphate) as a reference (ΦP = 0.074 in deaerated CH3CN).The DPA–OH concentration dependency of the upconversion emission was studied by varying the concentration of DPA–OH when all the other conditions were kept identical. As shown in Fig. 4, the upconversion fluorescence intensity increases with increasing the DPA–OH concentration. Obviously, the increased concentration of DPA–OH increases the collision chance between Pt–DPA and DPA–OH, which may further promote the intermolecular TTET between the Pt-complex and DPA–OH as well as the intermolecular triplet–triplet energy migration (TTEM) between the DPA chromophore of Pt–DPA and DPA–OH. To clarify the contribution from the intermolecular triplet–triplet energy transfer or the migration for the enhanced upconversion fluorescence, control experiments by using Pt–M instead of Pt–DPA were conducted. No upconversion fluorescence was observed in the Pt–M/DPA–OH systems ([Pt–M] = 1.0 × 10−5 M, [DPA–OH] = 0, 1.0, 1.4 × 10−4 M) by selective excitation of Pt–M with a 473 nm laser (Fig. S15†), suggesting that the effect of intermolecular triplet–triplet energy transfer between Pt–M and DPA–OH is minor, which can be rationalized to the short triplet lifetime of Pt–M and is validated by the transient absorption measurements (vide infra). The enhanced upconversion fluorescence with increasing the concentration of DPA–OH should be mainly affected by the intermolecular TTEM between the DPA chromophore of Pt–DPA and DPA–OH, which prolongs the triplet lifetime of DPA, thus advancing the TTA-UC process.
Studies on the TTA-UC process by transient absorption spectroscopy
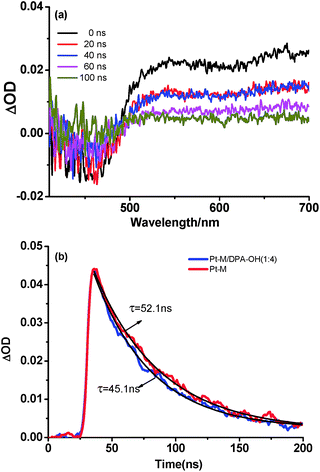
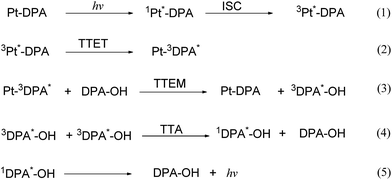
The details of the TTA-UC process were investigated by the transient absorption measurements. Pulse-laser photolysis of Pt–M, Pt–M/DPA–OH and Pt–DPA ([Pt–M], [Pt–DPA] = 1.0 × 10−4 M, [DPA–OH] = 4.0 × 10−4 M) was carried out in deaerated DMF by using 470 nm excitation light. Both transient absorption spectra of Pt–M and Pt–M/DPA–OH are similar and those in nanosecond scale are shown in Fig. S14† and 5a. The transient absorption in the region of 500–700 nm is assigned to the triplet state of Pt–M by referring to the literature.34 The negative band appeared in the transient absorption spectra is ground state bleaching with respect to its ground state absorption spectrum. Taking the transient absorption of the Pt–M and Pt–M/DPA–OH systems at 550 nm as a function of time gives the kinetic traces of the triplet state of Pt–M, which are shown in Fig. 5b. The kinetic traces can be well fitted monoexponentially, giving the triplet lifetime of Pt–M to be 45 and 52 ns in the presence and absence of DPA–OH, respectively. The transient absorption of Pt–M in the presence of DPA–OH decays slightly faster than that in the absence of DPA–OH, indicative of the occurrence of a low efficient triplet–triplet energy transfer between the Pt–M and DPA–OH. The efficiency of the intermolecular triplet–triplet energy transfer of the Pt–M/DPA–OH system is calculated to be 0.13. Under the experimental conditions, the short-lived triplet state of Pt–M can only encounter limited number of DPA–OH within its critical diffusion distance, giving a limited efficiency of the triplet–triplet energy transfer. The low efficiency of the intermolecular triplet–triplet energy transfer produces a small amount of the triplet state of DPA chromophore (3DPA*), consequently, resulting in no observation of the upconversion emission in the Pt–M/DPA–OH system. No transient absorption signal for Pt–DPA was captured in nanosecond scale, which indicates that the lifetime of the Pt-complex in Pt–DPA is less than the detection limit of the equipment (∼10 ns), giving an estimated efficiency of the intramolecular TTET in Pt–DPA is higher than 0.81.Pulse-laser photolysis of Pt–DPA (1.0 × 10−4 M) was further carried out in microsecond scale by using 470 nm excitation light. The transient absorption spectra in microsecond scale show a strong transient absorption band with a maximum at 440 nm (Fig. 6). The transient absorption spectrum is the same as that of DPA reported in the literature34 and is quenched in aerated solution, thus, it is assigned to 3DPA*. Upon irradiation of Pt–DPA at 470 nm, only the Pt-complex group absorbs the light; the detection of 3DPA* must be attributed to the triplet–triplet energy transfer from the Pt-complex group to the DPA chromophore. This gives a direct evidence for the triplet–triplet energy transfer in Pt–DPA. Similar transient absorption spectrum was obtained for the Pt–DPA/DPA–OH ([Pt–DPA] = 1.0 × 10−4 M, [DPA–OH] = 3.0 × 10−4 M) system (Fig. 6), demonstrating that 3DPA* can be generated by a triplet sensitization process. The optical density change of 3DPA* (ΔA(3DPA*)t) in Pt–DPA/DPA–OH is 0.08, which is slightly higher than that in Pt–DPA (0.07) under the same experimental conditions of Pt–DPA, indicating that a little more 3DPA* was generated in the Pt–DPA/DPA–OH system. The extra amount of 3DPA* can be ascribed to the intermolecular triplet–triplet energy transfer from the Pt-complex group of Pt–DPA to DPA–OH, which has been validated by the transient absorption measurements for the model systems of Pt–M and Pt–M/DPA–OH. No upconversion fluorescence from Pt–DPA can be observed in the absence of DPA–OH, although the generated 3DPA* is about 90% of that in the presence of DPA–OH. Therefore, the slight increment of 3DPA* in the presence of DPA–OH cannot be the main cause of the upconversion fluorescence.
 | ||
| Fig. 6 Transient absorption spectra observed upon photolysis of Pt–DPA and Pt–DPA/DPA–OH ([Pt–DPA] = 1.0 × 10−4 M, [DPA–OH] = 3.0 × 10−4 M) in deaerated DMF at the peak of the laser pulse (λex = 470 nm). Inset: decay trace for Pt–DPA and Pt–DPA/DPA–OH at 430 nm, and the blue line is the fit of decay trace for Pt–DPA with eqn (2). | ||
To understand the TTA process of 3DPA*, the kinetic traces of 3DPA* were further analyzed. The kinetic traces were obtained by taking the transient absorption of Pt–DPA and Pt–DPA/DPA–OH at 430 nm as a function of time, as shown in Fig. 6. 3DPA* in the Pt–DPA system undergoes two pathways: the first-order decay process including the phosphorescence and the intersystem crossing to the singlet ground state and the second-order TTA process. The time dependence of ΔA(3DPA*)t can be described by eqn (2).35,36 The situation in the Pt–DPA/DPA–OH system becomes complicated because of the existence of large amount of DPA–OH. Once the triplet state of Pt–3DPA* is formed, the triplet energy will mostly migrate to the DPA–OH to form 3DPA*–OH. Either Pt–3DPA* or 3DPA*–OH can undergoes the first-order and second-order photophysical processes and there is no proper description for the time dependence of ΔA(3DPA*)t in the Pt–DPA/DPA–OH system. The fraction (fTT) of 3DPA* decaying through the TTA pathway can be described as eqn (3):34,35
 | (2) |
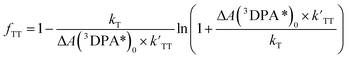 | (3) |
The analysis of the kinetic trace of 3DPA* in the Pt–DPA system demonstrates that the TTA process evidently exists in the Pt–DPA system, but no upconversion fluorescence was observed. To clarify the discrepancy between the insight of the photophysical processes and the observation of the upconversion fluorescence in the Pt–DPA system, the possibility of the formed singlet state of DPA (1DPA*) being quenched was analyzed. The absorption of the photosensitizer overlap the fluorescence of DPA and the free energy change involved in an electron transfer process between 1DPA* and the photosensitizer is also negative, indicating that the energy and electron transfer processes between 1DPA* and the photosensitizer is thermodynamically possible, which is further validated by the DPA fluorescence quenching experiments (Fig. S16†). The DPA fluorescence quenching experiments also indicated that the intramolecular quenching is more severe than the intermolecular one. Therefore, we infer that the quenching of 1DPA* by the photosensitizer is responsible for no observation of the upconversion fluorescence in the Pt–DPA system and for the low upconversion fluorescence quantum yield in the Pt–DPA/DPA–OH system. Similar situation was also observed in the photosensitizer-acceptor integrated TTA upconversion systems by other researchers.37,38 Therefore, to take full advantage of the intramolecular triplet–triplet energy transfer process to build an efficient TTA-UC system, a careful selection of photosensitizer and acceptor is also imperative to prevent quenching of the singlet state of the acceptor by the photosensitizer.
The main TTA-UC processes in the Pt–DPA/DPA–OH system upon excitation of the Pt-complex chromophore can be described as following and is shown in Scheme 2. (1) The Pt-complex chromophore of Pt–DPA is excited to form its singlet state (1Pt*–DPA) and goes to its triplet state (3Pt*–DPA) through the intersystem crossing process. (2) The triplet energy of 3Pt*–DPA transfers to the DPA chromophore of Pt–DPA to generate Pt–3DPA*. (3) The triplet energy migrates from Pt–3DPA* to DPA–OH producing 3DPA*–OH. (4) Two 3DPA*–OH molecules proceed the TTA process to generate a singlet state of DPA (1DPA*–OH) and a ground state of DPA (DPA–OH). (5) The radiative decay of 1DPA*–OH results in an upconverted fluorescence.
Conclusions and outlook
A model of dendritic structure compound Pt–DPA with two Pt-complex photosensitizer chromophores and two DPA groups covalently attached to the periphery and the core of the poly(aryl ether) dendrimer of generation 1 was synthesized. A TTA-UC system of Pt–DPA/DPA–OH is constructed by combining Pt–DPA with a dissociative acceptor DPA–OH. The acceptor covalently linked to the photosensitizer acts as an energy-relay to transfer the harvested energy to the dissociative acceptor, which further undergoes the TTA process. The upconversion fluorescence in the Pt–DPA/DPA–OH system was observed with quantum yield of 0.22% upon selective excitation of the Pt-complex chromophore with a 473 nm laser. The efficient intramolecular TTET process between the photosensitizer and the acceptor plays an important role for the TTA-UC system building with a short-lived triplet state photosensitizer, which facilitates the production of the triplet state of the acceptor, thus advancing the TTA-UC process. This work provides a new strategy for construction of the TTA-UC systems with short-lived triplet state photosensitizers.Developing photosensitizers with long lifetime of their triplet states to achieve efficient triplet–triplet energy transfer between the photosensitizer and the annihilator is still the main concern for constructing efficient TTA-UC systems. However, creation of photosensitizer array (such as polymeric or dendritic systems) may provide another possibility to extend stay of photosensitizers in their triplet state due to the intra-array energy migration. Furthermore, building a photosensitizer–annihilator array may also facilitate the triplet–triplet energy transfer from the photosensitizer to the annihilator, but the intra-array quenching of the upconversion fluorescence by the photosensitizer needs to be avoided by carefully selecting the photosensitizer and the annihilator. Any success of these studies can improve the TTA-UC systems.
Acknowledgements
We are grateful for financial support from the 973 program (Nos 2013CB834505, 2013CB834703), the National Natural Science Foundation of China (Nos 21273258, 21173245, 21233011, 21302196, and 21172229), and the Chinese Academy of Sciences (KGZD-EW-T05).Notes and references
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Y. Li, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- T. F. Schulze and T. W. Schmidt, Energy Environ. Sci., 2015, 8, 103–125 CAS.
- Q. Liu, B. R. Yin, T. S. Yang, Y. C. Yang, Z. Shen, P. Yao and F. Y. Li, J. Am. Chem. Soc., 2013, 135, 5029–5037 CrossRef CAS PubMed.
- P. F. Duan, N. Yanai, H. Nagatomi and N. Kimizuka, J. Am. Chem. Soc., 2015, 137, 1887–1894 CrossRef CAS.
- F. Marsico, A. Turshatov, R. Pekoz, Y. Avlasevich, M. Wagner, K. Weber, D. Donadio, K. Landfester, S. Baluschev and F. R. Wurm, J. Am. Chem. Soc., 2014, 136, 11057–11064 CrossRef CAS PubMed.
- A. Monguzzi, M. Frigoli, C. Larpent, R. Tubino and F. Meinardi, Adv. Funct. Mater., 2012, 22, 139–143 CrossRef CAS PubMed.
- B. Fuckel, D. A. Roberts, Y. Y. Cheng, R. G. C. R. Clady, R. B. Piper, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, J. Phys. Chem. Lett., 2011, 2, 966–971 CrossRef CAS.
- T. N. Singh-Rachford, A. Haefele, R. Ziessel and F. N. Castellano, J. Am. Chem. Soc., 2008, 130, 16164–16165 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino and F. Meinardi, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 155122 CrossRef.
- J. Z. Zhao, S. Ji and H. Guo, RSC Adv., 2011, 1, 937–950 RSC.
- J. Z. Zhao, W. H. Wu, J. F. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323–5351 RSC.
- D. S. Tyson, K. B. Henbest, J. Bialecki and F. N. Castellano, J. Phys. Chem. A, 2001, 105, 8154–8161 CrossRef CAS.
- J. F. Sun, W. H. Wu, H. M. Guo and J. Z. Zhao, Eur. J. Inorg. Chem., 2011, 3165–3173 CrossRef CAS PubMed.
- L. Liu, D. Huang, S. M. Draper, X. Yi, W. Wu and J. Zhao, Dalton Trans., 2013, 10694–10706 RSC.
- L. Ma, H. Guo, Q. Li, S. Guo and J. Zhao, Dalton Trans., 2012, 10680–10689 RSC.
- X. Yi, J. Zhao, J. Sun, S. Guo and H. Zhang, Dalton Trans., 2013, 2062–2074 RSC.
- S. K. Sugunan, U. Tripathy, S. M. K. Brunet, M. F. Paige and R. P. Steer, J. Phys. Chem. A, 2009, 113, 8548–8556 CrossRef CAS PubMed.
- T. N. Singh-Rachford, A. Nayak, M. L. Muro-Small, S. Goeb, M. J. Therien and F. N. Castellano, J. Am. Chem. Soc., 2010, 132, 14203–14211 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, J. Phys. Chem. Lett., 2010, 1, 195–200 CrossRef CAS.
- Y. Zeng, P. Li, X. Y. Liu, T. J. Yu, J. P. Chen, G. Q. Yang and Y. Li, Polym. Chem., 2014, 5, 5978–5984 RSC.
- J. Mei, Y. N. Hong, J. W. Y. Lam, A. J. Qin, Y. H. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- Y. Y. Cheng, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, Phys. Chem. Chem. Phys., 2010, 12, 66–71 RSC.
- X. Yu, X. Cao, X. Chen, N. Ayres and P. Zhang, Chem. Commun., 2015, 51, 588–591 RSC.
- J. Chen, S. Li, L. Zhang, B. Liu, Y. Han, G. Yang and Y. Li, J. Am. Chem. Soc., 2005, 127, 2165–2171 CrossRef CAS PubMed.
- J. Chen, J. P. Chen, S. Y. Li, L. Zhang, G. Q. Yang and Y. Li, J. Phys. Chem. B, 2006, 110, 4663–4670 CrossRef CAS PubMed.
- X. H. Zhang, Y. Zeng, T. J. Yu, J. P. Chen, G. Q. Yang and Y. Li, J. Phys. Chem. Lett., 2014, 5, 2340–2350 CrossRef CAS.
- H.-K. Yip, L.-K. Cheng, K.-K. Cheung and C.-M. Che, J. Chem. Soc., Dalton Trans., 1993, 2933–2938 RSC.
- Q.-Z. Yang, L.-Z. Wu, Z.-X. Wu, L.-P. Zhang and C.-H. Tung, Inorg. Chem., 2002, 41, 5653–5655 CrossRef CAS PubMed.
- S. K. Chattopadhyay, C. V. Kumar and P. K. Das, Chem. Phys. Lett., 1983, 98, 250–254 CrossRef CAS.
- F. Deng, J. R. Sommer, M. Myahkostupov, K. S. Schanze and F. N. Castellano, Chem. Commun., 2013, 49, 7406–7408 RSC.
- S. K. Sugunan, C. Greenwald, M. F. Paige and R. P. Steer, J. Phys. Chem. A, 2013, 117, 5419–5427 CrossRef CAS PubMed.
- J.-H. Kim and J.-H. Kim, J. Am. Chem. Soc., 2012, 134, 17478–17481 CrossRef CAS.
- P. Duan, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2013, 135, 19056–19059 CrossRef CAS PubMed.
- P. Jarosz, J. Thall, J. Schneider, D. Kumaresan, R. Schmehl and R. Eisenberg, Energy Environ. Sci., 2008, 1, 573–583 CAS.
- I. Carmichael and G. L. Hug, J. Phys. Chem. Ref. Data, 1986, 15, 1 CrossRef PubMed.
- J. Chen, S. Li, F. Gong, Z. Yang, S. Wang, H. Xu, Y. Li, J. S. Ma and G. Yang, J. Phys. Chem. C, 2009, 113, 11943–11951 CAS.
- G. Bergamini, P. Ceroni, P. Fabbrizzi and S. Cicchi, Chem. Commun., 2011, 47, 12780–12782 RSC.
- D. V. Kozlov and F. N. Castellano, Chem. Commun., 2004, 2860–2861 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of characterization of the synthetic compounds, transient absorption spectra of Pt–M, upconversion emission spectra of Pt–DPA/DPA–OH and Pt–M/DPA–OH, emission spectra of DPA–OH, Pt–DPA and Pt–M/DPA–OH. See DOI: 10.1039/c5ra12579k |
| This journal is © The Royal Society of Chemistry 2015 |