DOI:
10.1039/C5RA12570G
(Paper)
RSC Adv., 2015,
5, 80978-80989
Simultaneous removal of nickel and phosphorus from spent electroless nickel plating wastewater via calcined Mg–Al–CO3 hydroxides
Received
29th June 2015
, Accepted 9th September 2015
First published on 9th September 2015
Abstract
For electroless nickel plating wastewater, a novel approach for the simultaneous removal of nickel and phosphorus on calcined Mg–Al–CO3 hydroxides (CLDHs) is proposed. The dependence of the adsorption efficiency on several parameters, including initial ions' concentration, temperature, contact time and pH, has been investigated with batch experiments. The adsorption kinetics data of nickel and phosphorus could be well depicted by a pseudo-second-order model. Adsorption isotherms studies showed that the uptake of nickel and phosphorus on CLDHs followed Langmuir and Freundlich models, respectively, and that the maximum removal of nickel or phosphorus was up to 22.87 or 761.5 mg g−1. Thermodynamic analyses implied that the adsorption process of nickel or phosphorus on CLDHs was spontaneous and endothermic. Further, the possible mechanisms were explored in: low concentration solutions, CLDHs took part in reconstitution involving the isomorphous substitution of nickel at the magnesium sites in sheets and by the concomitant utilization of phosphorus by the generated superficial sheets; and in high concentration solutions, the CLDHs rebuilding hydrotalcite structures were influenced and formed mixed metal salts of phosphites, hydroxides, and hypophosphites, which were attributed to the presence of plentiful phosphorus and brought about the reduced uptake of nickel.
1. Introduction
Due to the excellent physicochemical properties of coatings (e.g. homogeneity, high hardness, wearability and corrosion resistance), the electroless nickel (EN) plating technique has been widely applied in automotive, aerospace, plastic, machinery, packaging and electronic computer industries.1,2 Specifically, in the fastest-growing electronics industries, the EN plating technique enables the metalizing of plastic or glass parts surface to decorate or improve functionality, which is closely related to our daily necessities, for instance cell phone' shells. For the electroless nickel plating technique, the chemical reaction can be expressed as:| | |
3NaH2PO2 + NiSO4 → Ni + 2H2 + 2P + 4NaH2PO3 + Na2SO4
| (1) |
During the electroless Ni–P plating process, both concentrations of phosphites and organic compounds gradually accumulate to an unavailable level as the cycle continues.3 Plenty of spent wastewater is produced with high quantities of nickel ions and phosphites and a small amount of hypophosphites and organic acids. Like other potentially toxic metals, nickel cannot be decomposed, and the toxicity is gradually increased because of accumulation in living organisms and consequently it is biomagnified in the food chain, which could result in cancer.4,5 On the other hand, although phosphorus is a crucial element for organisms' growth in ecosystems and environments, excess phosphate can cause aquatic eutrophication, especially in enclosed systems, leading to an overgrowth of aquatic plants, the depletion of dissolved oxygen and even the death of fish and other aquatic organisms.6 Phosphate can also improve the contents of Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) in wastewater, making disposal more difficult. Therefore, the removal of nickel and phosphorus from the spent EN plating wastewater using an effective and robust technique is of utmost importance.
Several approaches have been proposed to treat spent EN plating wastewater such as alkaline precipitation, ion exchange, electrodialysis, adsorption, membrane filtration and solvent extraction.7,8 Among these, alkaline precipitation is the most common method; however, the high buffer capacity from organic substances demands excessive amounts of chemicals to neutralize the alkalinity. The precipitated sludge contains extremely hazardous waste of nickel hydroxides and thus must be further controlled. It is worth noting that the abovementioned methods are mainly focused on nickel removal, whereas less attention has been paid to the high concentration of phosphite. Herein, it is essential to develop a cost-effective and facile technique to simultaneously remove nickel and phosphorus from EN plating wastewater.
Layered double hydroxides (LDHs), a class of anionic clays with a 2D-nanostructure,9 can be expressed with the general formula of [M1−x2+Mx3+(OH)2] (An−)x/n·yH2O, where M2+ is the divalent cation (Mg2+, Ni, Cu2+); M3+ is the trivalent cation (Al3+, Fe3+, Cr3+); An− is the n-valent anion (inorganic, organic, complex and bioorganic), and x can have various values between 0.17 and 0.33.10 Because of the special structures of LDHs, Zhou et al.11 and Xu et al.12 utilized the ions in electroplating wastewaters to form LDH precipitates to purify themselves. Although certain positive results have been achieved, the key focus was only to remove metal ions. Recently, Zhu et al. simultaneously recovered Ni, P and S from spent electroless nickel plating by forming graphene/NiAl hydroxides;13 however, the process was complicated and required extra H2O2, thus inducing a high cost. Previous studies have demonstrated that LDHs and calcined LDHs (CLDHs) can be used for removing anions and metallic cations. Moreover, CLDHs (M1−x2+Mx3+O1+x/2) possess higher adsorption capacity than LDHs owing to the unique property known as the “memory effect”, which can spontaneously reconstruct the layered structure via rehydration, the isomorphous substituting of the sites of metallic elements in sheets and incorporating anions into interlayers from the aqueous solution.14–16 Literature research has indicated that massive study is directed toward treating simulated wastewater containing anions via CLDHs, yet few studies exist on CLDHs and metal-containing industrial wastewater. To the best of our knowledge, no study has been done on the co-adsorption of metallic cations (Ni2+) and anions (H2PO3−/H2PO2−) in spent EN plating wastewater.17
In this study, a novel feasible approach using CLDHs as a low-cost adsorbent for the co-treatment of nickel and phosphorus in electroless nickel plating wastewater is presented. To explore the adsorption performance of CLDHs, the effects of several parameters, such as the ions' initial concentration, temperature, contact time and pH, were investigated. Using X-ray diffraction (XRD), Fourier transform infrared spectra (FTIR), Scanning Electron Microscopy (SEM), Electron dispersive X-ray analysis (EDX) and X-ray photoelectron spectroscopy (XPS) technologies, the possible adsorption mechanism was further elucidated.
2. Experimental
2.1. Materials
The chemicals, Mg(NO3)2·6H2O, Al(NO3)3·9H2O, NH4HCO3 and NH3·H2O were of analytical purity and used as received. The industrial spent EN plating wastewater containing high quantities of nickel ions (Ni2+) and phosphites (H2PO3−), and a small amount of hypophosphites (H2PO2−) and organic acids (lactic acid and acetic acid) were used for the stock solutions. The initial concentration of nickel (Ni) or phosphorus (P, phosphites and hypophosphites) was about 2.5 g L−1 and 200 g L−1, respectively. Deionized water was used in all experiments.
2.2. Synthesis of the LDHs and CLDHs
Mg3Al–CO3 hydrotalcites (LDHs) were prepared by the co-precipitation method at constant pH (10 ± 0.5).18 One solution (400 mL) contained Mg(NO3)·6H2O (0.12 mol) and Al(NO3)3·9H2O (0.04 mol). A second solution (250 mL) contained 0.015 mol NH4HCO3 and 0.48 mol NH3·H2O. At room temperature, the two solutions were simultaneously added dropwise to 400 mL of deionized water with continuous stirring. The resulting slurry was stirred to age for a specified period. The final precipitate was filtered, washed, and dried during 24 h at 80 °C to obtain LDHs. Calcined Mg3Al–CO3 hydrotalcites (CLDHs) were obtained by calcining LDHs in a muffle furnace at 450 °C for 2 h and placed in desiccators for the following experiments.
2.3. Adsorption experiments
Batch experiments were conducted with the industrial spent EN plating wastewater to investigate the sorption capacities of CLDHs for Ni and P. All the experiments were performed in 250 mL conical flasks and shaken in a temperature-controlled orbital shaker (stirring speed of 100 rpm) at different temperatures. A constant mass 0.05 g of CLDHs was added to the diluting solution (100 mL) with different initial concentrations of Ni and P. The initial pH values of the solutions were not adjusted to avoid other new ions affecting the Ni or P uptake. After a period of time, 2 mL of supernatant was filtered by 0.22 μm membrane. The residual Ni concentration was obtained by a flame atomic absorption spectrophotometer. For P, it was obtained by the molybdenum-blue ascorbic acid method using a UV-visible spectrophotometer at a wavelength of 700 nm.19
The removal percentage of Ni or P onto CLDHs was calculated according to eqn (2):20
| | |
Removal (%) = 100(Co − Ct)/Co
| (2) |
Ni or P adsorbed by CLDHs was calculated by the following equation:
where
Co,
Ct and
Ce are the initial, time
t and equilibrium concentration of Ni or P (mg L
−1);
qt is the adsorptive capacity of adsorbent at time
t (mg g
−1),
qe =
qt when the adsorption reaches equilibrium;
V is the volume of solution (L); and
m is the mass of adsorbent (g).
2.3.1. Kinetics study. To find out the adsorption equilibrium time, different time intervals ranging from 0 to 600 min were set to test at 25, 35, 45 or 55 °C. The kinetics models were adopted during 300 min at 35 °C and 45 °C. To investigate Ni and P, the reaction solutions were obtained by diluting stock solutions 125 times (20.00 mg L−1 Ni + 1600 mg L−1 P) and 1000 times (200.0 mg L−1 P + 2.500 mg L−1 Ni), respectively.
2.3.2. Isotherm study. The adsorption isotherms were investigated with a range of different initial concentrations of Ni and P at 25, 35, 45 and 55 °C. The reaction solutions were prepared as follows: (1) Ni (15.00 to 25.00 mg L−1) + P (1200 to 2000 mg L−1), (2) Ni (0.63 to 7.500 mg L−1) + P (50.00 to 600.0 mg L−1). The experiments were carried out for a period of 300 min to allow the uptake of Ni or P up to equilibrium.
2.3.3. Effect of initial ion concentration. The effect of initial Ni or P concentration was studied for 5 h by diluting the stock solutions as different proportions at 55 °C. The solutions were prepared as follows: (1) Ni (5.00 to 32.50 mg L−1) + P (400.0 to 2600 mg L−1), (2) Ni (0.25 to 37.50 mg L−1) + P (20.00 to 3000 mg L−1).
2.3.4. Effect of pH. The study was performed at 35 °C for 5 h with different dilution solutions: (1) 20.00 mg L−1 Ni + 1600 mg L−1 P (dilution rate of 125 times), (2) 2.500 mg L−1 Ni + 200.0 mg L−1 P (dilution rate of 1000 times), (3) 0.250 mg L−1 Ni + 20.00 mg L−1 P times (dilution rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times). The pH value was determined by a PHS-3C meter at different time intervals (0 to 300 min).
000 times). The pH value was determined by a PHS-3C meter at different time intervals (0 to 300 min).
2.4. Characterization of samples before and after the adsorption
Powder X-ray diffraction (XRD) data were obtained with a Rigaku D/MAX-RA instrument using Cu Kα radiation (λ = 0.154184 nm) at 40 kV and 50 mA. Fourier transform infrared spectra (FTIR) were obtained on an AVATAR 370 spectrometer in the 4000–400 cm−1 wavenumber range with the KBr disk method. Scanning Electron Microscopy (SEM) images with electron dispersive X-ray analysis (EDX) were carried out on the Hitachi S-4800 microscope with EDAX apparatus. The X-ray photoelectron spectroscopy (XPS) was obtained using an ESCALAB 250 spectrometer (ThermoFisher Scientific) with an Al Kα X-ray source (0.60 eV) and the optimal energy resolution was less than 0.45 eV.
3. Results and discussion
3.1. Effect of contact time
The effect of contact time on the adsorption of Ni and P on CLDHs was evaluated to determine the equilibration time and to investigate the adsorption process. Plots of Ni or P removal rate versus the change in contact time (0–600 min) at different temperatures are presented in Fig. 1. Roughly, there was a monotonic increase in the removal efficiency of Ni or P on CLDHs with time for all temperatures, up to the adsorption saturation. The removal efficiency of Ni or P on CLDHs increased with increasing temperature at the same contact time. The uptake rate of Ni or P was rapid at the starting stage and then suffered a slow process, suggesting one adsorption process was not solely controlled by the external mass transfer adsorption process.21 The changes of Ni or P removal rates from 300 to 600 min were slight, thus the adsorption capacity of CLDHs was basically represented in the first 300 min. The study also indicated the adsorption of Ni or P on CLDHs was limited and irreversible. Therefore, a contact time of 300 min was applied for the further studies as the adsorption equilibrium time.
 |
| | Fig. 1 Effect of contact time of Ni or P on CLDHs at different temperatures ((a) [Ni + P] = 20 mg L−1 + 1600 mg L−1, (b) [Ni + P] = 2.5 mg L−1 + 200 mg L−1, [CLDHs] = 0.5 g L−1). | |
3.2. Adsorption kinetics
To determine the rate constants and explore the adsorption mechanism of Ni or P on CLDHs, kinetics studies were performed over 300 min. The experimental data were modeled with pseudo-first-order,22 pseudo-second-order23 and intraparticle diffusion24 models, which could be described by the linearized form:| |
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (4) |
| | |
t/qt = 1/k2qe2 + t/qe
| (5) |
where k1 (min−1), k2 (g mg−1 min−1) and ki (mg g−1 min−0.5) are the rate constants of the pseudo-first-order, pseudo-second-order and intraparticle diffusion models, respectively. The relevant kinetics parameters calculated from the slopes and intercepts of linear plots are presented in Table 1.
Table 1 Kinetic models for the adsorption of Ni and P on CLDHs at 35 °C and 45 °C
| |
T (°C) |
qe,exp (mg g−1) |
Pseudo-first-order |
Pseudo-second-order |
Particle diffusion |
| qe,cal (mg g−1) |
k1 (×10−2) (min−1) |
R2 |
qe,cal (mg g−1) |
k2 (×10−4) (g mg−1 min−1) |
R2 |
ki (mg g−1 min−0.5) |
R2 |
| Ni |
35 |
17.33 |
14.77 |
1.453 |
0.9760 |
19.36 |
13.87 |
0.9968 |
0.9716 |
0.9135 |
| 45 |
21.46 |
13.89 |
1.350 |
0.9195 |
22.84 |
19.68 |
0.9990 |
1.168 |
0.8318 |
| P |
35 |
146.4 |
115.9 |
1.138 |
0.9595 |
167.2 |
1.283 |
0.9983 |
8.632 |
0.9173 |
| 45 |
188.0 |
133.0 |
1.357 |
0.9360 |
206.2 |
1.625 |
0.9988 |
10.83 |
0.8606 |
In Table 1, the equilibrium adsorption capacities (qe,cal) of the pseudo-second-order model were close to the experimental results (qe,exp) at high correlation coefficients R2 (>0.99) compared to the other two models. Consistent result could be obtained from Fig. 3, wherein the fitting curves of the pseudo-second-order model are presented. Herein, the pseudo-second-order model could fairly well describe the uptake of Ni and P onto CLDHs, revealing the chemical sorption process.25 In addition, the rate constant k2 and equilibrium capacity qe,cal for Ni or P increased with increasing temperatures, demonstrating that raising temperatures were favorable for adsorption progress.
 |
| | Fig. 2 Effect of initial concentration of Ni (left) or P (light) on the removal efficiency and adsorption capacity ([CLDHs] = 0.5 g L−1, t = 300 min, T = 55 °C). | |
 |
| | Fig. 3 Kinetics of Ni (a) or P (b) adsorption on CLDHs at different temperatures and data fitting by pseudo-first-order, pseudo-second-order, and particle diffusion models. ((a) [Ni + P] = 20 mg L−1 + 1600 mg L−1, (b) [Ni + P] = 2.5 mg L−1+ 200 mg L−1, [CLDHs] = 0.5 g L−1, t = 300 min). | |
3.3. Effect of ion initial concentration
The removal performance of CLDHs for Ni or P is shown in Fig. 2 as a function of initial ion concentration. Fig. 1 shows that the adsorption equilibrium could be reached within 300 min, thus the contact time was set to 300 min. It can be seen from Fig. 2 that increased initial ion concentrations would result in a reduction in removal efficiencies of Ni and P. In terms of Ni, it could be effectively removed from solution with an initial concentration lower than 10.00 mg L−1. Likewise, as the initial concentration of P was under 20.00 mg L−1, the removal efficiency remained above 90%. Moreover, the removal efficiency turned lower than 50% as the initial concentrations of Ni and P went higher than 25.00 mg L−1 and 400.00 mg L−1, respectively. Therefore, the initial concentrations of Ni (20.00 mg L−1) and P (200.00 mg L−1), wherein the approximate removal efficiency (over 50%) could be obtained, were chosen for the kinetics study.
As seen in Fig. 2, with increasing initial concentration, the adsorption amounts of Ni firstly rose and then dropped, whereas the adsorption quantity of P strictly increased. With the same dilution ratio of stock solutions, the adsorption quantity of CLDHs for P was considerably higher compared with Ni, thus elucidating the higher affinity of CLDHs for P than for Ni. The co-existing high concentration of P might have a negative effect on Ni removal. Considering the appropriate removal efficiency and adsorption capacity, the initial concentrations of Ni and P were in the range of 15.00–25.00 mg L−1 and 50.00–600.00 mg L−1, respectively, which were the concentrations applied in the adsorption isotherm study.
3.4. Adsorption isotherm
An adsorption isotherm is usually applied in describing the interaction between adsorbents and adsorbates and can be used to evaluate the adsorption capacity of adsorbents.26 Freundlich and Langmuir isotherm models are most frequently applied for analyzing equilibrium sorption data.
The Langmuir isotherm model assumes that the adsorbed layer is monolayer and all adsorption sites are equal and homogeneous.27 The equation can be given as follows:28
| | |
Ce/qe = 1/(qmKL) + Ce/qm
| (7) |
where
qm (mg g
−1) is the maximum adsorption capacity and
KL (L mg
−1) is the Langmuir constant related to the energy of adsorption.
The Freundlich model is based on the assumption that a multilayer sorption occurs on heterogeneous surface sites with different bond energies.29 The liner equation of Freundlich model can be expressed as follows:
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n KF + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (8) |
where
KF (mg g
−1)(L mg
−1)
n and
n are Freundlich constants related to the adsorption intensity.
Adsorption isotherms of Ni or P on CLDHs were fitted with Langmuir and Freundlich models at various temperatures, as shown in Fig. 4. The parameters of the two models are summarized in Table 2. Furthermore, for Ni adsorption, it is obvious that all the correlation coefficient (R2) values exceed 0.99 derived from the Langmuir model, suggesting that the Langmuir model could better describe Ni adsorption than the Freundlich model. Whereas the results from the Freundlich model were more properly attributed to the higher R2 of the Freundlich model than those of the Langmuir model in the case of P adsorption. It was concluded that the adsorption process of Ni was homogeneous, whereas P was heterogeneous.
 |
| | Fig. 4 Sorption isotherm of Ni (left) or P (right) using CLDHs at 25, 35, 45, or 55 °C. Solid lines and dotted lines represent predicted data by the Langmuir model and Freundlich model, respectively, and the symbols are the experimental data ([CLDHs] = 0.5 g L−1, t = 300 min). | |
Table 2 Langmuir and Freundlich isotherm parameters for the adsorption of Ni and P on CLDHs
| |
T (°C) |
Langmuir adsorption model |
Freundlich adsorption model |
| Qma (mg g−1) |
KL (×10−3)(L mg−1) |
R2 |
KF (mg g−1)(L mg−1)n |
n |
R2 |
| Ni |
25 |
17.33 |
854.7 |
0.9994 |
12.35 |
10.22 |
0.9482 |
| 35 |
19.15 |
813.6 |
0.9995 |
13.22 |
9.362 |
0.9326 |
| 45 |
21.35 |
768.6 |
0.9989 |
14.36 |
8.536 |
0.9695 |
| 55 |
22.87 |
761.5 |
0.9985 |
14.95 |
7.753 |
0.9454 |
| P |
25 |
520.8 |
3.673 |
0.9639 |
9.634 |
1.742 |
0.9972 |
| 35 |
684.9 |
3.026 |
0.9549 |
8.363 |
1.584 |
0.9987 |
| 45 |
1063 |
2.168 |
0.8469 |
7.209 |
1.422 |
0.9940 |
| 55 |
2273 |
1.406 |
0.6718 |
6.380 |
1.228 |
0.9935 |
In Fig. 4, the adsorption capacity of CLDHs was improved with increasing equilibrium concentrations of Ni or P, attributed to the ascending initial concentrations. The rising temperatures also promoted the adsorption capacity of CLDHs for Ni or P, which was consistent with the kinetics study. Therefore, the maximum adsorption quantity could be obtained at 55 °C with maximal initial concentrations of 25.00 (Ni) and 600.0 (P) mg L−1. The adsorption capacity of CLDHs was 22.87 mg g−1 for Ni based on the Langmuir model and 761.5 mg g−1 for P based on the Freundlich model.
The sorption capacity of Ni or P on CLDHs was compared with some other adsorbents and the results are summarized in Table 3.6,16,19,30–37 On the basis of the results, hydroxides have a higher adsorption capacity for Ni or P than other adsorbents, but calcined hydroxides possessed higher superiority than LDHs in single ion adsorption system. This indicated that CLDHs were a type of potential adsorbent for Ni and P removal. The sorption capacity of Ni on CLDHs decreased in the present study (22.87 mg g−1) compared to the single ion adsorption system (361.1 mg g−1),34 whereas P increased. This also indicated that the co-existing high concentration P had a negative influence on the uptake of Ni removal, as the effect of ion initial concentration results shows.
Table 3 Comparative studies on the uptake capacities of calcined Mg–Al hydroxides with other reported adsorbents for Ni and P
| Adsorbates |
Adsorbents |
T (°C) |
qm (mg g−1) |
References |
| Ni |
Calcined Bofe bentonite clay |
20 |
1.910 |
29 |
| Row doum seed coats |
Room temperature |
3.240 |
30 |
| Multiwalled carbon nanotubes |
25 |
6.090 |
31 |
| Immobilized sericite beads |
Room temperature |
10.74 |
32 |
| Hydroxides |
— |
4.110–42.84 |
16 |
| Calcined Mg–Al hydroxides |
50 |
361.1 |
33 |
| Calcined Mg–Al hydroxides |
55 |
22.87 |
Present study |
| P |
Nano-bimetal ferrites |
45 |
13.50 |
6 |
| Activated aluminum oxide |
25 ± 1 |
20.88 |
34 |
| Bephos™ |
25 ± 1 |
26.50 |
35 |
| Zn–Al hydroxides |
30 |
76.10 |
36 |
| Calcined Zn–Al hydroxides |
30 |
232.0 |
36 |
| Calcined Mg–Al hydroxides |
25 |
147.4 |
37 |
| Calcined Mg–Al hydroxides |
55 |
761.5 |
Present study |
3.5. Effect of temperature
The plots of qt versus t (Fig. 3) and qe versus Ce (Fig. 4) at varying temperatures implied that the increased temperatures promoted Ni or P removal. It could be speculated that the uptake of Ni or P on CLDHs was endothermic, with more active sites of adsorbent surfaces at higher temperatures.
To further understand the effect of temperature, the thermodynamic parameters, including the change in the Gibbs free energy (ΔG°, J mol−1), enthalpy (ΔH°, J mol−1) and entropy (ΔS°, J mol−1 K−1), were analyzed. These parameters can provide in-depth information regarding the inherent energy and structural changes and can contribute to evaluate the orientation and feasibility of the adsorption reaction.38 Thermodynamic equations can be expressed as follows:30
| |
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD KD
| (9) |
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD = ΔS°/R − ΔH°/RT KD = ΔS°/R − ΔH°/RT
| (11) |
where
R is the universal gas constant (8.314 × 10
−3 kJ mol
−1 K
−1);
T is the absolute temperature (K) and
KD is the adsorbate distribution coefficient and is calculated by
eqn (9),
The values of the thermodynamics parameters reported in Table 4 were obtained from the linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K versus 1/T (Fig. 5). The values of ΔH° and ΔS° were positive, and the value of ΔG° was negative for all the experiments, which was obvious. The positive ΔH° implied that the endodermic nature of the adsorption process16 improved with rising temperatures, adsorption capacity and adsorption rate of CLDHs for Ni or P. The positive value of ΔS° showed the increased randomness, ascribed to the shift of partial adsorbates from the solutions to the adsorbent–solution interfaces during the adsorption processes. Therefore, the concentration of Ni or P in solution decreased with the increased time and temperature. With a rise of temperature, the negative ΔG° decreased, suggesting a spontaneous adsorption process. Furthermore, the increased temperature was favorable for Ni or P adsorption, which was in accordance with the previous studies.
K versus 1/T (Fig. 5). The values of ΔH° and ΔS° were positive, and the value of ΔG° was negative for all the experiments, which was obvious. The positive ΔH° implied that the endodermic nature of the adsorption process16 improved with rising temperatures, adsorption capacity and adsorption rate of CLDHs for Ni or P. The positive value of ΔS° showed the increased randomness, ascribed to the shift of partial adsorbates from the solutions to the adsorbent–solution interfaces during the adsorption processes. Therefore, the concentration of Ni or P in solution decreased with the increased time and temperature. With a rise of temperature, the negative ΔG° decreased, suggesting a spontaneous adsorption process. Furthermore, the increased temperature was favorable for Ni or P adsorption, which was in accordance with the previous studies.
Table 4 Thermodynamic parameters of Ni or P adsorption by CLDHs as a function of temperature
| |
ΔG° (kJ mol−1) |
ΔH° (kJ mol−1) |
ΔS° (kJ mol−1) |
| 298 K |
308 K |
318 K |
328 K |
| Ni |
−6.675 |
−7.032 |
−7.397 |
−7.792 |
4.394 |
0.03712 |
| P |
−0.04041 |
−0.2069 |
−0.4053 |
−0.5920 |
5.481 |
0.01851 |
 |
| | Fig. 5 Plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD versus 1/T for estimation of the thermodynamic parameters for the adsorption of Ni (left) or P (right) on CLDHs. KD versus 1/T for estimation of the thermodynamic parameters for the adsorption of Ni (left) or P (right) on CLDHs. | |
3.6. Effect of pH
The pH value can affect the chemical properties of adsorbents and adsorbates, therefore influencing the adsorption process of Ni or P on CLDHs. As illustrated in Fig. 6, with increasing time, pH values dramatically increased at the beginning, followed by a slow increase before a near balanced condition, which was consistent with the results of Fig. 1 and 3, suggesting that pH variation affects the removal of Ni or P owing to the released hydroxyls (OH−) of adsorbents. In adsorption processes, pH values within the range of 5–10 were low 12.5 (pHPZC of Mg3Al CLDHs),39 implying the positive charges of adsorbents surfaces. CLDHs could rehydrate in solution leading to the release of OH− with increased pH. Herein, plentiful OH− was produced on the surfaces of adsorbents and was partially transferred to solution, causing rising alkalinity gradients from the liquid phases to the solid–liquid interfaces. Under the effect of the alkalinity gradient, Ni was easily transferred from the liquid phases to solid–liquid interfaces in the form of hydroxides. Simultaneously, anions of solutions could gather toward the positively charged surfaces of adsorbents by electrostatic attraction. Ionic pollutants escaped from solutions to adsorbents surfaces purifying the wastewater. Because of the “memory effect”,14 Ni could occupy Mg sites in sheets and participate in refactoring lattices of LDHs by isomorphic substitution,34,40 and anions could concomitantly incorporate into interlayers by electrostatic attraction.
 |
| | Fig. 6 Effect of pH on the adsorption of Ni and P on CLDHs in different concentrations of dilute solution ((a) [Ni + P] = 0.25 mg L−1 + 20 mg L−1, (b) [Ni + P] = 2.5 mg L−1+ 200 mg L−1, (c) [Ni + P] = 20 mg L−1 + 1600 mg L−1, [CLDHs] = 0.5 g L−1, t = 300 min, T = 35 °C). | |
In addition, phosphorus mainly existed in the form of H2PO3− (with few H2PO2−) in the electroless nickel plating wastewater. The dissociation equilibrium of H3PO3 or H3PO2 in aqueous solution is pH-related, which can be presented as:
| | |
H3PO3 ⇌ H2PO3− + H, pKa = 1.3
| (13) |
| | |
H2PO3− ⇌ HPO32− + H+, pKa = 6.6
| (14) |
| | |
H3PO2 ⇌ H2PO2− + H+, pKa = 11
| (15) |
In Fig. 6(b) and (c), the pH values basically changed in the range of 5–7. Herein, plentiful H2PO3− (with few H2PO2−) was the dominant species in adsorption processes. The affinities of LDHs were in the sequence H2PO3−/H2PO2− > CO32− > SO42− > OH−/lactic acid/acetic acid,41,42 thus phosphorus could be first removed from solution and gathered at the surfaces of adsorbents by electrostatic attraction.
3.7. Characterization
Fig. 7 represents the XRD patterns of Mg–Al–CO3 LDHs, CLDHs, CLDHs after the adsorption of Ni and P with low initial concentrations ([Ni + P] = 0.2500 mg L−1 + 20.00 mg L−1) (R1-CLDHs), and CLDHs after the adsorption of Ni and P with high initial concentrations ([Ni + P] = 20 mg L−1 + 1600 mg L−1) (R2-CLDHs). The X-ray patterns of LDHs demonstrated not only sharp and symmetrical peaks (003, 006, 110 and 113), but some other asymmetrical peaks of hydrotalcites structures as well, indicating well-crystallized LDHs. Moreover, a hexagonal lattice with an R3m rhombohedral symmetry could be indexed through the patterns of LDHs, which further proved the successful synthesis of hydroxides materials. The peaks of hydrotalcites structures disappeared in the XRD patterns of CLDHs, indicating that the calcinations intensely destroyed the original hydrotalcites structures resulting in the collapse of the sheet structures and the formation of a mixed oxide Mg(Al)O with MgO-periclase structures (JCPDS 45-0496).43,44 The patterns of R1-CLDHs were similar to the patterns of LDHs, with decreasing relative intensities of peaks, inferring the formation of hydrotalcites after adsorption. This might show that Ni is indeed incorporated into the hydrotalcites lattices. It was interesting that the 2θ values of the characteristic diffraction peak (003) were similar in Fig. 6(a) and (b), revealing the interlayer spacing was not changed after adsorbing H2PO3− or H2PO2−. Therefore, it might be a reasonable explanation that the adsorbed phosphorous ions were mainly utilized by the superficial sheets of the reconstructed LDHs, and many OH−, CO32− or SO42− were inserted into the interlayers of internal sheets. When the patterns of R2-CLDHs contained some unobvious peaks attributed to the low crystallinity, which was different from the patterns of LDHs indicating the non-layered structures of R2-CLDHs. It was reasonable to speculate that a large amount of H2PO3− (or H2PO2−) in solution affected the reconstructions of LDH structures, forming new adsorption products. Without LDH structures, Ni could not be incorporated into the hydrotalcites lattices, inducing decreased adsorption capacity. It was indicated that Ni uptake was affected by the presence of plentiful P, as the effects of the initial ion concentration results show. Besides, via the JCPDS database, the MgHPO3 phase (JCPDS 72-1173) was observed for the R2-CLDH sample. The HPO32− generated was attributed to the release of abundant OH−, leading to the hydrolysis reaction of H2PO3−. The low crystallinity showed that the adsorption products in high concentration solutions were mixtures. Combining the results of the pH effect, the mixtures mainly contained metal (Mg, Al or Ni) salts of phosphites, hypophosphites and hydroxides.
 |
| | Fig. 7 XRD patterns (left) and FT-IR (right) for LDHs (a), CLDHs (b), R1-CLDHs (c) and R2-CLDHs (d). | |
FTIR analysis can provide direct information about surface and bulk species.45 The FTIR spectra of LDHs, CLDHs, R1-CLDHs and R2-CLDHs are illustrated in Fig. 7. Broad bands in the range of 3400–3500 cm−1 (O–H stretching vibration) and weak bands at about 1600 cm−1 (O–H bending vibration) demonstrated the presence of interlaminar water molecules.46 All the spectra contained a strong bond at about 1400 cm−1, attributed to the asymmetric stretching vibration of CO32− of the interlayers. The peaks between 400 and 700 cm−1 were assigned to metal–oxygen–metal stretching.47 After adsorption of Ni and P, new bands appeared at about 790 and 1120 cm−1, as can be seen in Fig. 6(b) and (c), which might be indicative of Ni–O stretching and P–O vibration, respectively.48 These indicated CLDHs could indeed simultaneously adsorb Ni and P.
To further investigate the adsorption mechanism of Ni or P, the morphology of LDHs, CLDHs, R1-CLDHs and R2-CLDHs and some corresponding chemical compositions are presented in Fig. 8. The SEM images showed an alveolate-like morphology with plentiful flakiness for LDHs, displaying the thin crystals of LDHs.49 It can be further observed in Fig. 8(b) that the flakiness structures existing were aggregative and rough without abundant morphologies, showing the collapsed layer structures of CLDHs by calcination. In addition, the morphology of the R1-CLDHs was similar to LDHs, and the EDX spectra were presented for the components of LDHs (C, H, O, Mg and Al) and the adsorbates (Ni and P). With low contents of Ni and P, it was observed that the layered structures of hydrotalcites was reconstructed via isomorphously substituting Mg with Ni, utilizing H2PO3−/H2PO2− to balance the positive charges of the superficial sheets and intercalating OH−, CO32− or SO42− into interlayers of internal sheets according to the XRD results. In high concentration solutions, more Ni and P could be absorbed by CLDHs, as indicated by the EDX spectra of R1-CLDHs and R2-CLDHs. However, the SEM image of R2-CLDHs was without an obvious alveolate-like morphology and was evidently different from that of LDHs or R1-CLDHs, which showed that for the morphology, new adsorption products were generated and did not belong to LDHs. This was in accordance with the XRD results.
 |
| | Fig. 8 The SEM image of LDHs (a), CLDHs (b), R1-CLDHs (c) or R2-CLDHs (d), and the corresponding EDX spectrum of R1-CLDHs (c) or R2-CLDHs (d). | |
The XPS technique was employed for an in-depth analysis of the adsorption processes. Fig. 9(a) shows the surface compositions of LDHs, R1-CLDHs, R2-CLDHs and CLDHs. The carbon, oxygen, Mg and Al could be observed in all spectra. New peaks of Ni 2p and P 2p on R1-CLDHs or R2-CLDHs could be detected, revealing Ni or P adsorption on the CLDHs. Specifically, there was no obvious peak for Ni 2p on R1-CLDHs, indicating a low adsorption quantity due to the low initial concentration of Ni. In Fig. 9(b), the main contribution of the Ni 2p core level spectrum was centered at 856.8 eV, which was assigned to Ni2+ interacting possibly with H2PO3− or H2PO2−.50,51 Regarding the P 2p, two deconvoluted peaks appeared after adsorption, verifying P existed in the spent EN plating wastewater in two forms, namely H2PO3− and H2PO2−.50,52 The peak, as obvious is centered at the high binding energy side was predominantly ascribed to the high proportion of H2PO3− in the total phosphorus content. Besides, P still existed in the original valence state after adsorption, which implied that they were used to balance the positive charges of the produced sheets during the CLDHs' refactoring processes, and the adsorbed contents of P were not high enough to change the LDHs structures. Two peaks of R2-CLDHs were upward shifted compared to R1-CLDHs indicating the change of the bonding environment of H2PO3− or H2PO2− species. Mg3Al CLDHs, one type of mixed metal oxide (Mg(Al)O), when in contact with the solutions could form Mg or Al hydroxides on the surfaces, due to the ionization effect together with Ni hydroxides of the surfaces and could then generate the single octahedral sheets.53 According to the multilayer sorption obtained from the Freundlich model, large quantities of H2PO3− or H2PO2− on the surfaces of adsorbents occupied at the adsorption sites of surface sheets and interlayers by linking the generated single sheets, and excess parts replaced the –OH sites of the sheets. Most of the H2PO3− or H2PO2− incorporated into the interlayers caused sheets distortions, and thus the sheets could not stack together to form the LDH structures. Because abundant OH− were released from the adsorbents surfaces and as large quantities of H2PO3− or H2PO2− on the surfaces of the adsorbents existed, finally they could combine with ionized Mg2+, Al3+ or Ni2+ to form metal salts. These results are in accordance with the XRD and SEM results.
 |
| | Fig. 9 (a) X-ray photoelectron spectra of LDHs, R1-CLDHs, R2-CLDHs, and CLDHs, (b) Ni 2p of R2-CLDH core level spectrum after adsorption on CLDHs, and (c) comparison of the XPS P 2p peaks between R1-CLDHs (c) and R2-CLDHs. | |
3.8. Adsorption mechanism
Based on the abovementioned studies, the adsorption mechanisms could be speculated as follows: (1) for low concentration solutions, CLDHs took place in rehydration and rebuilt the LDH structures. Most Ni was incorporated into sheets and P was mainly utilized to balance the positive charges of the superficial sheets; (2) for high concentration solutions, first, Ni adhered to the surfaces of the adsorbents via the alkalinity gradient in the form of hydroxide precipitations, and P accumulated on the positively charged adsorbent surfaces through electrostatic attraction. Then, abundant H2PO3− or H2PO2− occupied the interlayer sites and replaced the –OH sites of the sheets, leading to twisty sheets and affecting the formation of LDH structures. Finally, mixed metal (Mg, Al or Ni) salts of phosphites, hydroxides or hypophosphites were produced. These reactions might be expressed as eqn (16) and (17) and are depicted in Scheme 1.| | |
Mg1−xAlxO1+x/2 + yNi2+ + (x + 2y)H2PO3−/H2PO2− + (m + 1 + x/2)H2O → Mg1−xNiyAlx(OH)2H2PO3−/H2PO2(x+2y)−·mH2O + xOH−
| (16) |
| | |
Mg(Al)O + Ni2+ + H2PO3−/H2PO2− + H2O + CO2 → (Mg/Al/Ni)(H2PO3/H2PO2)n + (Mg/Al/Ni)(OH)n + (Mg/Al/Ni)2(CO3)n + OH−
| (17) |
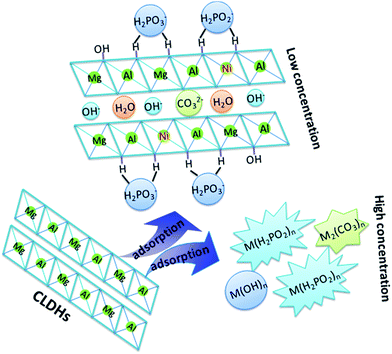 |
| | Scheme 1 Schematic of probable adsorption mechanisms. | |
4. Conclusions
The CLDHs demonstrated an excellent purification effect for spent EN plating wastewater with respect to the co-removal of nickel and phosphorus. It was found that the adsorption process of Ni and P on CLDHs was endothermic and spontaneous, together with increasing the randomness of the system. A pseudo-second-order equation well explained the kinetic data and revealed the possibility of chemisorptions. Moreover, adsorption isotherms could be fitted by Langmuir and Freundlich models, showing the different uptake processes of Ni and P. As a result, CLDHs could rebuild hydrotalcite structures utilizing the ions of Ni and P in low concentration solutions, whereas new mixed metal salts were generated in high concentration solutions. In addition, the removal of Ni was negatively impacted, because of the adsorption of large amounts of P. This study demonstrated that CLDHs possessed vast application potential in treating spent EN plating wastewater with high efficiency, easy operation and at low cost.
Acknowledgements
The current study was financially supported by the Science and Technology Development Plan of Shandong Province of China (No. 2012GGE27098) and the Independent Innovation Projects of Important Key Technology, Shandong Province (No. 2014GJJS0501).
References
- S. Tengsuwan and M. Ohshima, J. Supercrit. Fluids, 2012, 69, 117–123 CrossRef CAS PubMed.
- T. Zhai, X. Lu, G. Cui, G. Wu, J. Qu and Y. Tong, J. Mater. Chem. C, 2013, 1, 5149 RSC.
- Y. J. Shih, C. P. Lin and Y. H. Huang, Sep. Purif. Technol., 2013, 104, 100–105 CrossRef CAS PubMed.
- M. Jiang, X. Jin, X. Lu and Z. Chen, Desalination, 2010, 252, 33–39 CrossRef CAS PubMed.
- K. Dermentzis, J. Hazard. Mater., 2010, 173, 647–652 CrossRef CAS PubMed.
- Y. Tu and C. You, Chem. Eng. J., 2014, 251, 285–292 CrossRef CAS PubMed.
- M. Hunsom, K. Pruksathorn, S. Damronglerd, H. Vergnes and P. Duverneuil, Water Res., 2005, 39, 610–616 CrossRef CAS PubMed.
- Y. Huang and M. Tanaka, J. Hazard. Mater., 2009, 164, 1228–1235 CrossRef CAS PubMed.
- X. Yuan, J. Wang, C. Zhou, Q. Tang and X. Rao, Chem. Eng. J., 2013, 221, 204–213 CrossRef CAS PubMed.
- T. Kwon, G. A. Tsigdinos and T. J. Pinnavaia, J. Am. Chem. Soc., 1988, 110, 3653–3654 CrossRef CAS.
- J. Zhou, Y. Wu, C. Liu, A. Orpe, Q. Liu, Z. Xu, G. Qian and S. Qiao, Environ. Sci. Technol., 2010, 44, 8884–8890 CrossRef CAS PubMed.
- Y. Xu, J. Zhang, J. Zhou, C. Chen, Q. Liu, G. Qian and Z. P. Xu, Chem. Eng. J., 2013, 215–216, 411–417 CrossRef CAS PubMed.
- X. Zhu, F. Xie, J. Li and G. Jin, J. Environ. Chem. Eng., 2015, 3, 1055–1060 CrossRef CAS PubMed.
- O. D. Pavel, R. Bîrjega, M. Che, G. Costentin, E. Angelescu and S. Serban, Catal. Commun., 2008, 9, 1974–1978 CrossRef CAS PubMed.
- K. H. Goh, T. T. Lim and Z. Dong, Water Res., 2008, 42, 1343–1368 CrossRef CAS PubMed.
- X. Liang, Y. Zang, Y. Xu, X. Tan, W. Hou, L. Wang and Y. Sun, Colloids Surf., A, 2013, 433, 122–131 CrossRef CAS PubMed.
- L. Fang, W. Li, H. Chen, F. Xiao, L. Huang, P. E. Holm, H. C. B. Hansen and D. Wang, RSC Adv., 2015, 5, 18866–18874 RSC.
- T. Kameda, M. Umetsu and T. Yoshioka, New J. Chem., 2015, 39, 4078–4085 RSC.
- P. Cai, H. Zheng, C. Wang, H. Ma, J. Hu, Y. Pu and P. Liang, J. Hazard. Mater., 2012, 213–214, 100–108 CrossRef CAS PubMed.
- R. Wang, T. Wen, X. Wu and A. Xu, RSC Adv., 2014, 4, 21802 RSC.
- Y. Li, Q. Yue and B. Gao, J. Hazard. Mater., 2010, 178, 455–461 CrossRef CAS PubMed.
- K. K. C. Namasivayam, Carbon, 1999, 37, 79–84 CrossRef.
- Y. S. Ho and G. Mckay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- M. Jansson-Charrier, E. Guibal, J. Roussy, B. Delanghe and P. Le Cloirec, Water. Res., 1996, 30, 465–475 CrossRef CAS.
- D. Wan, H. Liu, R. Liu, J. Qu, S. Li and J. Zhang, Chem. Eng. J., 2012, 195–196, 241–247 CrossRef CAS PubMed.
- Z. Zhang, M. Liao, H. Zeng, S. Xu, X. Liu, J. Du, P. Zhu and Q. Huang, Appl. Clay Sci., 2014, 102, 246–253 CrossRef CAS PubMed.
- J. C. T. H. Genc-Fuhrman and D. McConchie, Environ. Sci. Technol., 2004, 38, 2428–2434 CrossRef.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- Y. Cengeloglu, A. Tor, M. Ersoz and G. Arslan, Sep. Purif. Technol., 2006, 51, 374–378 CrossRef CAS PubMed.
- M. G. Vieira, A. F. Neto, M. L. Gimenes and M. G. da Silva, J. Hazard. Mater., 2010, 177, 362–371 CrossRef CAS PubMed.
- M. El-Sadaawy and O. Abdelwahab, Alexandria Eng. J., 2014, 53, 399–408 CrossRef PubMed.
- N. T. Abdel-Ghani, G. A. El-Chaghaby and F. S. Helal, J. Adv. Res., 2014, 6, 405–415 CrossRef PubMed.
- C. Jeon and J. Cha, J. Ind. Eng. Chem., 2014, 24, 107–112 CrossRef PubMed.
- M. Sun, Y. Xiao, L. Zhang, X. Gao, W. Yan, D. Wang and J. Su, Chem. Eng. J., 2015, 272, 17–27 CrossRef CAS PubMed.
- J. Xie, Y. Lin, C. Li, D. Wu and H. Kong, Powder Technol., 2015, 269, 351–357 CrossRef CAS PubMed.
- M. Zamparas, M. Drosos, Y. Georgiou, Y. Deligiannakis and I. Zacharias, Chem. Eng. J., 2013, 225, 43–51 CrossRef CAS PubMed.
- J. Zhou, S. Yang, J. Yu and Z. Shu, J. Hazard. Mater., 2011, 192, 1114–1121 CrossRef CAS PubMed.
- A. Ş. Yargıç, R. Z. Yarbay Şahin, N. Özbay and E. Önal, J. Cleaner Prod., 2015, 88, 152–159 CrossRef PubMed.
- L. Xiao, W. Ma, M. Han and Z. Cheng, J. Hazard. Mater., 2011, 186, 690–698 CrossRef CAS PubMed.
- T. H. Kim, W. J. Lee, J. Y. Lee, S. M. Paek and J. M. Oh, Dalton Trans., 2014, 43, 10430–10437 RSC.
- S. Miyata, Clays Clay Miner., 1983, 31, 305–311 CAS.
- T. Wu, L. Mao and H. Wang, RSC Adv., 2015, 5, 23246–23254 RSC.
- D. Lan, L. Ma, Y. Chun, C. Wu, L. Sun and J. Zhu, J. Catal., 2010, 275, 257–269 CrossRef CAS PubMed.
- M. Hájek, P. Kutálek, L. Smoláková, I. Troppová, L. Čapek, D. Kubička, J. Kocík and D. N. Thanh, Chem. Eng. J., 2015, 263, 160–167 CrossRef PubMed.
- T. Wang, Z. Cheng, B. Wang and W. Ma, Chem. Eng. J., 2012, 181–182, 182–188 CrossRef CAS PubMed.
- R. Shan, L. Yan, Y. Yang, K. Yang, S. Yu, H. Yu, B. Zhu and B. Du, J. Ind. Eng. Chem., 2015, 21, 561–568 CrossRef CAS PubMed.
- H. Zaghouane-Boudiaf, M. Boutahala and L. Arab, Chem. Eng. J., 2012, 187, 142–149 CrossRef CAS PubMed.
- Q. Yu, Y. Zheng, Y. Wang, L. Shen, H. Wang, Y. Zheng, N. He and Q. Li, Chem. Eng. J., 2015, 260, 809–817 CrossRef CAS PubMed.
- Q. Guo and E. J. Reardon, Appl. Clay Sci., 2012, 56, 7–15 CrossRef CAS PubMed.
- J. A. Cecilia, A. Infantes-Molina, E. Rodríguez-Castellón and A. Jiménez-López, J. Catal., 2009, 263, 4–15 CrossRef CAS PubMed.
- N. Escalona, J. Ojeda, P. Baeza, R. García, J. M. Palacios, J. L. G. Fierro, A. L. Agudo and F. J. Gil–Llambías, Appl. Catal., A, 2005, 287, 47–53 CrossRef CAS PubMed.
- H. Song, M. Dai, H. Song, X. Wan and X. Xu, Appl. Catal., A, 2013, 462–463, 247–255 CrossRef CAS PubMed.
- Y. Xiao, M. Sun, L. Zhang, X. Gao, J. Su and H. Zhu, RSC Adv., 2015, 5, 28369–28378 RSC.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times). The pH value was determined by a PHS-3C meter at different time intervals (0 to 300 min).
000 times). The pH value was determined by a PHS-3C meter at different time intervals (0 to 300 min).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t
qe − k1t

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n
KF + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD
KD
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD = ΔS°/R − ΔH°/RT
KD = ΔS°/R − ΔH°/RT
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K versus 1/T (Fig. 5). The values of ΔH° and ΔS° were positive, and the value of ΔG° was negative for all the experiments, which was obvious. The positive ΔH° implied that the endodermic nature of the adsorption process16 improved with rising temperatures, adsorption capacity and adsorption rate of CLDHs for Ni or P. The positive value of ΔS° showed the increased randomness, ascribed to the shift of partial adsorbates from the solutions to the adsorbent–solution interfaces during the adsorption processes. Therefore, the concentration of Ni or P in solution decreased with the increased time and temperature. With a rise of temperature, the negative ΔG° decreased, suggesting a spontaneous adsorption process. Furthermore, the increased temperature was favorable for Ni or P adsorption, which was in accordance with the previous studies.
K versus 1/T (Fig. 5). The values of ΔH° and ΔS° were positive, and the value of ΔG° was negative for all the experiments, which was obvious. The positive ΔH° implied that the endodermic nature of the adsorption process16 improved with rising temperatures, adsorption capacity and adsorption rate of CLDHs for Ni or P. The positive value of ΔS° showed the increased randomness, ascribed to the shift of partial adsorbates from the solutions to the adsorbent–solution interfaces during the adsorption processes. Therefore, the concentration of Ni or P in solution decreased with the increased time and temperature. With a rise of temperature, the negative ΔG° decreased, suggesting a spontaneous adsorption process. Furthermore, the increased temperature was favorable for Ni or P adsorption, which was in accordance with the previous studies.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD versus 1/T for estimation of the thermodynamic parameters for the adsorption of Ni (left) or P (right) on CLDHs.
KD versus 1/T for estimation of the thermodynamic parameters for the adsorption of Ni (left) or P (right) on CLDHs.






