Simultaneous determination of vasicine and its major metabolites in rat plasma by UPLC-MS/MS and its application to in vivo pharmacokinetic studies
Abstract
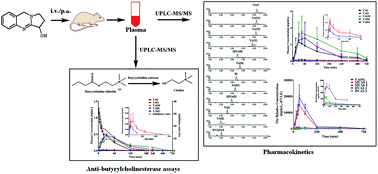
An efficient and sensitive ultra-performance liquid chromatography-tandem mass spectrometry method has been developed and validated to simultaneously determine and quantify vasicine (VAS) and its major metabolites including vasicinone (VAO), 9-oxo-1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-yl hydrogen sulfate (VAOS), 1,2,3,9-tetrahydro-pyrrolo[2,1-b]quinazolin-3-yl hydrogen sulfate (VASS), 1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-β-D-glucuronide (VASG), vasicinol (VASL) and vasicinolone (VAOL) using pseudoephedrine as the internal standard in rat plasma. The chromatographic separation was conducted on a HSS T3 column (100 mm × 2.1 mm, 1.8 μm) with the gradient elution using a mobile phase of methanol–0.1% formic acid in water at a flow rate of 0.4 mL min−1 for 7 min. The tandem mass spectrometric detection was conducted using multiple reaction monitoring (MRM) by positive electrospray ionization (ESI). The corresponding lower limits of quantitation (LLOQ) of the method were 0.73, 0.80, 0.75, 0.80, 0.82, 0.87, 0.82 ng mL−1 for VAO, VAOS, VASS, VASG, VAS, VASL and VAOL, respectively. The within- and between-run precision for all analytes were less than 7.66% and 12.30%, respectively. The recovery for all analytes was between 85.89% and 114.58%, and the matrix effects for all analytes were not observed. By the UPLC-MS/MS method, the relative quantitation of five metabolites of 9-oxo-1,2,3,9-tetrahydropyrrolo[2,1-b]quinazolin-3-β-D-glucuronide (VAOG), hydroxylation–acetylation products of VAS (HVAS1 and HVAS2) and methylation–acetylation products of VAS (MVAS1 and MVAS2) were achieved by standard curves derived from the urine sample with treatment by VAS as a reference substance, in which the considerable target metabolites were included. This method was successfully applied to pharmacokinetic studies of VAS and its metabolites in rats. The activity of the components in plasma after intravenous administration of VAS (2 mg kg−1) was evaluated by in vitro anti-butyrylcholinesterase assays. The results indicated that in vivo butyrylcholinesterase inhibitive activities were mainly due to the different concentrations of prototype VAS and a few other metabolites.

 Please wait while we load your content...
Please wait while we load your content...