Novel synthesis and cytotoxic activity of 1,4-disubstituted 3-methylidene-3,4-dihydroquinolin-2(1H)-ones†
Abstract
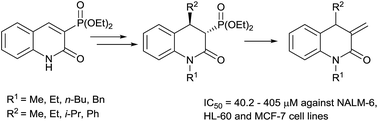
A novel, efficient synthesis of 1,4-disubstituted 3-methylidene-3,4-dihydrodihydroquinolin-2(1H)-ones was accomplished via a three step reaction sequence comprising N-alkylation of 3-diethoxyphosphorylquinolin-2(1H)-one, Michael addition of various Grignard reagents to N-alkylated 3-diethoxyphosphorylquinolin-2(1H)-ones and a Horner–Wadsworth–Emmons reaction of the obtained adducts with formaldehyde. Effective synthesis of the starting 3-diethoxyphosphorylquinolin-2(1H)-one was also developed. Furthermore, the obtained 3-methylidene-3,4-dihydroquinolin-2(1H)-ones were evaluated for their cytotoxic activity.

 Please wait while we load your content...
Please wait while we load your content...