Improvement of lignin yield and purity from corncob in the presence of steam explosion and liquid hot pressured alcohol†
Xianhong Ouyanga,
Wenya Wang*a,
Qipeng Yuana,
Shuangxi Lib,
Qiuxiang Zhangb and
Pengxiang Zhaoc
aCollege of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: wangwy@mail.buct.edu.cn
bCollege of Mechanic and Electronic Engineering, Beijing University of Chemical Technology, Beijing 100029, China
cState Grid Energy Conservation Service Co., Ltd., Beijing 100031, China
First published on 10th July 2015
Abstract
Non-food biomass such as corncob is a very abundant and promising feedstock for sustainable energy production in China. Many studies have focused on overcoming the barrier of saccharification of corncob. However, lignin was not well exploited as the renewable aromatic resource in nature since a simple single protocol usually cannot facilitate bio-recalcitrance or advanced methods (ionic liquid etc.) hardly met the economic viability on a plant scale. Here we provided an easy combined process which could reap pure, high-yield and low molecular weight lignin. Moreover, cellulose and hemicellulose were easy digested into value-added products (xylose and glucose). The results showed that: (1) the lignin rich fraction was pure and of a high-yield, main linkages was cleaved during the combined process; (2) hemicellulose was utterly removed and transformed into xylose; (3) the cellulose rich fraction was easy to digestion enzymatically and the glucose conversion was 95.7% of the theoretical value.
1. Introduction
Corn yield (21.85 million tons) represented the greatest proportion of the total grain yield in 2013 in China.1 Usually the rest of the corncob is burned and this treatment has negative effects on the air environment, which is one of the reasons for smog.2 Corncob is rich in lignocellulose which has huge potential for producing glucose, xylose, and aromatic chemicals. Many technologies were studied on utilising the single component of corncob, such as using ionic liquids to enhance the enzymatic hydrolysis of cellulose,3 microwaves were employed to enhance conversion of hemicellulose into xylose.4 Lignin is the second most abundant source of renewable carbon accounting for 15% to 30% of dry weight of lignocellulose.5 Abundant lignin in corncob was interlaced with a carbohydrate complex but was not well exploited. High quality lignin, as a renewable material, has great potential to produce basic industrial aromatic chemicals.6 Pulp and paper industries generate a great amount of lignin currently as a by-product of delignification and biofuel refinery is expected to produce more lignin in the future.7 However, this lignin could only be exploited in low value applications8 and to generate heat9 nowadays. Consequently, improving the added-value worth of lignin has been attracting more attention in lignocellulose biorefinery.10It has been demonstrated that lignin constitutes the main barrier for the enzymatic hydrolysis of cellulose in lignocellulose and the removal of lignin could facilitate the enzymatic efficiency.11 Up to now, the alkali treatment was the most extensive-used method for delignification. However, alkali delignification is considered to be environmental unfriendly, it produced a variety of pollutants, especially the inorganic salts, which were unable to be utilized by biodegradation.12 Ionic liquid attracted attention as a green promising solvent for biomass pre-treatment.13 Currently, 1-ethyl-3-methylimidazolium acetate ([Emim][CH3COO]) was considered to be the best solvent which could selectively extract lignin from lignocellulose. However, the lignin yield was low and the molecular weight was higher relatively.14
In the 1990s, the ethanol delignification was developed and reached a pre-commercial stage as a green delignification method,15 which was characterized with the advantages of easy recovery of ethanol, less production of pollutants and elimination of the odour when compared to alkali delignifying methods. Moreover, organosolv lignin was considered to be high quality because of its high purity and primarily unaltered structure. It could be applied to the fields of adhesives,16 nanostructured films17 and copolymers.18 However, ethanol lignin could not meet the industrial requirements in comparison with the conventional kraft lignin in terms of economic performance because of low yield of lignin caused by the structure complexity of lignocellulose. A feasible solution for overcoming this was to modify this process to produce profitable products.
More recently, a clean process for xylose production in pilot scale by using screw-steam-explosive extruder was developed in our previous study. More than 90% of hemicellulose was degraded and converted into mono xylose, a high-valued fine chemicals.19 In the present study, the ethanol solution was applied to extract organosolv lignin from the steam explosion residue (SER) and the higher yield and purity of organosolv ethanol lignin was obtained in the presence of diluted acid steam explosion (DASE) and liquid hot pressured alcohol delignification (LHPAD).
In order to improve the value-added worth of lignin, this study aimed to develop a clean, effective and economic process for organosolv ethanol lignin preparation in combination of steam explosion and ethanol delignifying process.
2. Experimental
2.1 Raw materials and the combined process of DASE and LHPAD
Dried untreated corncob (UC) was from Shandong province and chipped to a size of 2 cm and stored at room temperature. Lignin was isolated by the procedure described at Fig. 1. The sample was immersed in H2SO4 solution (0.5% w/w) overnight and drained by squeeze. It was treated by steam explosion in a screw-steam-explosive extruder at the optimal operating parameters (5 min and 1.5 MPa) and the component of elution was mono xylose as described in our previous work.19 The dried SER was treated with ethanol aqueous (20/80 v/v) at 160 °C for 120 min. The solid to liquid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. This experiment was carried out in a 0.5 L stainless steel high-pressure reactor. After alcohol delignification, the insoluble residue was filtered and washed with the same ethanol aqueous three times, which was rich in cellulose and named as cellulose rich fraction (CRF). The filtrate was collected and concentrated, 3 volumes of acid water (pH = 2) were dropped to precipitate the lignin rich fraction (LRF), which was collected by centrifugation and air dried at 40 °C for 24 hours.
10. This experiment was carried out in a 0.5 L stainless steel high-pressure reactor. After alcohol delignification, the insoluble residue was filtered and washed with the same ethanol aqueous three times, which was rich in cellulose and named as cellulose rich fraction (CRF). The filtrate was collected and concentrated, 3 volumes of acid water (pH = 2) were dropped to precipitate the lignin rich fraction (LRF), which was collected by centrifugation and air dried at 40 °C for 24 hours.
2.2 Chemical composition analysis
The cellulose, hemicellulose, lignin and ash were determined by the NREL method (determination of structural carbohydrates and lignin in biomass).20 Dried samples (300 mg) was hydrolyzed using 3 mL 72% H2SO4 for 60 min at 30 °C in a water bath with intermittent shocking every 5–10 minutes. Add 84 mL water to dilute H2SO4 to 4% and hydrolyze at 121 °C for 60 min in an autoclave. The dried residue of hydrolysis was acid insoluble lignin (AIL). The hydrolysate was analyzed by UV spectrophotometer (Shimadzu UV2450) to determine acid soluble lignin (ASL) and carbohydrate was determined by High Performance Liquid Chromatography (Hitachi, Tokyo, Japan) equipped with a Sugar-pak 1 column (Waters, Milford, MA, USA). The AIL was placed in a muffle furnace at 575 °C to determine the ash content.2.3 Fourier transform infrared spectroscopy
The FTIR spectrums of all samples were recorded in a Nicolet 6700 spectrophotometer in the scan range from 400 cm−1 to 4000 cm−1 with a resolution of 4 cm−1. A 200 mg KBr disc containing 1 mg sample was used in FTIR measurement.2.4 X-ray diffraction
XRD was carried out in a Rigaku D/Max 2500 VB2+/PC, with 5° min−1 scan speed and 5–50° scan scope. Copper radiation (k = 0.154185 nm) was generated at a voltage of 40 kV. The crystal index was calculated by the following equation:
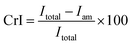 | (1) |
2.5 HSQC of LRF
Acetylation was frequently performed on lignin before NMR analysis (ESI†). The HSQC spectrum of LRF was done by a Bruker AV 600 spectrometer. 100 mg acetylated lignin was dissolved in 0.5 mL DMSO-d6. The spectral widths were 7788 Hz and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165 Hz for 1H and 13C dimensions respectively. The number of collected complex points was 2048 for 1H dimension with a recycle delay of 1.5 s. The number of transients was 64 and 256 time increments were recorded in 13C dimension. The 1JCH used was 145 Hz. Data was analyzed by Bruker Topspin software. The DMSO peak (δC/δH = 39.5/2.49) was used as an internal chemical shift.
165 Hz for 1H and 13C dimensions respectively. The number of collected complex points was 2048 for 1H dimension with a recycle delay of 1.5 s. The number of transients was 64 and 256 time increments were recorded in 13C dimension. The 1JCH used was 145 Hz. Data was analyzed by Bruker Topspin software. The DMSO peak (δC/δH = 39.5/2.49) was used as an internal chemical shift.
2.6 Gel permeation chromatograms
LRF sample was dissolved in 1 mL 1% NaOH aqueous solution and then diluted to proper concentration with deionized water for GPC analysis. The Waters GPC system (Milford, Massachusetts, USA) equipped with UV detector was employed for molecular weight determination. The column was TSK G3000PWxl. The different molecular-weight (Mw = 210, 4300, 6800, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 17
000, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 32
000, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 150
000, 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) poly styrenesulfonic acid salt (purchased from Sigma-Aldrich) was hired as standards. The experiment was operated at room temperature and eluted with 0.1 M NaNO3 solution at a flow rate of 1 mL min−1 and 50 μL sample was injected into the GPC system.
000) poly styrenesulfonic acid salt (purchased from Sigma-Aldrich) was hired as standards. The experiment was operated at room temperature and eluted with 0.1 M NaNO3 solution at a flow rate of 1 mL min−1 and 50 μL sample was injected into the GPC system.
2.7 Enzymatic hydrolysis of CRF
The enzymatic hydrolysis of UC, SER and CRF was carried out in 50 mM citrate buffer (pH = 4.8) with 5 g substrates in 100 mL buffer at 50 °C, 150 rpm for 72 h. The cellulase (Novozyme Cellic CTec2) activity was 206 FPU mL−1 and the enzyme loading was 18 FPU g−1 cellulose. The results of enzymatic hydrolysis were determined by HPLC described in 2.2.3. Results and discussion
3.1 Chemical compositions of samples
The chemical compositions of each fraction were given in Table 1. The chemical compositions of UC were 34.24% cellulose, 29.27% hemicellulose, 17.98% acid soluble lignin and 11.33% acid insoluble lignin. Based on the chemical composition of LRF in Table 1, it indicated that purity of lignin was improved by the combination of DASE and LHPAD. The lignin purity reached 93.57%, which was primarily consisted of acid insoluble lignin. Acid soluble lignin was dissolved and could not be recovered by acid precipitate since the existence of diluted acid, as a catalyst, providing hydrion and cleaving the α- and β-aryl ether linkages of ASL during the DASE.21 The lignin yield was improved from 6.81% to 40.94% (Fig. S1†). It was higher than that from previous Alcell process significantly.22 The hemicellulose composition after DASE was 1.11%, demonstrating that most hemicellulose was depolymerized and removed from the lignocellulose during the DASE via autohydrolysis reaction.23 Therefore, the high lignin yield could attribute to the physical crushing ability of DASE, offering more accessible area for alcohol to enrich the lignin after removal of the hemicellulose component. The cellulose (66.58%) in CRF increased twofold after combined process, which indicated that most ASL and partial AIL was dissolved and removed from SER, while cellulose was retained in the solid phrase. Ethanol process with acid catalyst, such as H2SO4, HCl and phosphoric acid, was frequently used to extract lignin from different biomass. However, lignin purity from switchgrass was approximate 70–80% (with 0.9 w/w% sulfuric acid) and this lignin was contaminated by carbohydrates.24 Other organosolv lignin extraction after steam explosion was complex and it needed subsequent lignin purification process involved many operations and chemicals (acetic acid, dioxane, ethyl ether etc.).25 Lignin has complex and irregular structures crosslinking with carbohydrates by tight physical bonding and chemical linkages. Single process is too powerless to overcome lignin–carbohydrate recalcitrance, leading to low purity and low yield. For example, ionic liquid was considered to be the green solvent for lignin, however, only up to 20 wt% lignin isolated from pine kraft pulp could dissolve in [Hmim][CF3SO3].26 During the combined process of DASE and LHPAD, amorphous hemicellulose and part of cellulose was separated by DASE, which contributed to the higher lignin purity and yield than ethanol process with acid catalyst obviously.3.2 FTIR analysis
FTIR spectroscopy was applied to compare the molecular conformation changes of samples after different treatments. All FTIR data was recorded in Fig. 2 and corresponding band assignments were showed in Table 2. The bands at 3421 cm−1 and 2900 cm−1 were attributed to stretch of –OH groups and C–H stretching, which existed in cellulose, hemicelluloses and lignin. The signals at 1739 cm−1, 1705 cm−1 and 1639 cm−1 in UC were assigned to p-coumaryl ester group, which is the typical sign of the natural lignin. After combined treatments, carbonyl group was detected in the sample of SER, LRF and CRF, characterized with presence of signal peak at 1705 cm−1 while absence of signal peaks at 1739 cm−1 and 1639 cm−1. This change could be assigned to dehydration reaction of lignin during steam explosion, which generated ketone groups27 (Fig. S2†). Obvious characteristic peaks of the aromatic skeletal vibrations at 1604 cm−1, 1513 cm−1 and 1425 cm−1 were observed in all samples, which indicated the existence of lignin in them. The signal at 1166 cm−1 was the sign for H/S/G lignin and this signal in LRF was more intensive than that in other samples, which agreed with the chemical composition analysis of LRF, i.e. lignin in LRF with higher purity. Moreover, the FTIR data of LRF was in agreement with reported literatures that G unit and S unit usually were incorporated in herbaceous plants with significant amounts of H unit. According to F. Carrillo,28 the signals at 1161 cm−1 and 1056 cm−1 were assigned to the C–O anti-symmetrical bridge stretching and C–O–C pyranose ring skeletal vibration. The signals at 1161 cm−1 and 1056 cm−1 could be observed in UC and SER, while in CRF the signal at 1161 cm−1 was disappeared and the signal at 1056 cm−1 was weakened. Apparent peak was detected at 897 cm−1, showing the glycosidic C1–H deformation with ring vibration, which indicated the existence of β-glycosidic linkages in the UC, SER and CRF. The peak at 1105 cm−1 was detected in UC while absent in other samples, indicating the change of cellulose structure caused by the combined process: the tendency of conformation change from cellulose I in UC to cellulose II in SER and CRF.29| Band (cm−1) | Assignment | UC | SER | CRF | LRF |
|---|---|---|---|---|---|
| 3500–3300 | O–H stretching | 3421 | 3421 | 3421 | 3421 |
| 2918 | Aliphatic | 2891 | 2902 | — | 2917 |
| 2850–2830 | C–H symmetric stretching in methyl and methylene group | — | — | 2850 | — |
| 1720–1680 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in unconjugated ketone, carbonyl, and ester groups O stretching in unconjugated ketone, carbonyl, and ester groups |
— | 1702 | 1704 | 1704 |
| 1660–1640 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in conjugated p-substituted aryl ketones O stretching in conjugated p-substituted aryl ketones |
1633 | 1631 | 1631 | 1630 |
| 1610–1590 | Aromatic skeletal vibrations plus C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching O stretching |
1606 | 1604 | 1604 | 1604 |
| 1515–1505 | Aromatic skeletal vibrations | 1515 | 1513 | 1513 | 1513 |
| 1470–1460 | C–H deformations (asymmetric in –CH3 and –CH2–) | — | 1454 | 1460 | 1456 |
| 1420 | Aromatic skeletal vibrations combined with C–H in plane deformations | 1427 | 1429 | 1425 | 1425 |
| 1365–1370 | Aliphatic C–H stretching in CH3 (not in OMe) and phenolic OH | 1373 | 1371 | 1362 | 1363 |
| 1267 | G unit breathing | — | 1261 | 1263 | 1259 |
| 1166 | HSG unit | — | 1164 | 1168 | 1166 |
| 1161 | Anti-symmetrical bridge C–O–C stretching | 1160 | — | — | — |
| 1130–1120 | S unit | — | — | 1126 | 1124 |
| 1056 | C–O stretching | 1054 | 1058 | 1056 | — |
| 1033 | Aromatic C–H in plane deformation (G + S units) | — | 1031 | 1033 | 1031 |
| 897 | β-Linkage of cellulose | 898 | 898 | 898 | — |
| 833 | C–H out of plane in positions 2, 5 and 6 (G + S unit) | 833 | 833 | 833 | 833 |
3.3 HSQC spectrum of LRF
Lignin has an immense potential to produce aromatic chemicals and meet energy fuel requirements. The specific utilization of lignin was depended on its unique structure. Consequently, LRF structure was characterized by HSQC. In the aromatic area (Fig. 3A), signals of syringyl (S), guaiacyl (G), p-hydroxyphenyl (H) units could be observed. The S unit showed signals for the C2,6–H2,6 correlation at δC/δH 104.1/6.67; the G unit showed signals for the C2–H2 correlation at δC/δH 111.3/7.02, C5–H5 correlation at δC/δH 115.4/6.69, C6/H6 correlation at δC/δH 119.0/6.79 and the oxidized G unit had signals for the C6–H6 correlation at δC/δH 123.3/7.60. The signals corresponding to C2,6–H2,6 correlation in H unit were observed at δC/δH 128.2/7.16. These results agreed with FTIR analysis indicating that H/S/G lignin was obtained after steam explosion and liquid hot alcohol delignification. In addition, the p-coumaroylated substructures could also be observed at δC/δH 144.1/7.43, δC/δH 130.2/7.48 and δC/δH 115.4/6.78 for its C7–H7, C2,6–H2,6 and C3–H3, C5–H5 correlations, respectively. The S/G ratio of LRF was 1/72 according to their relative abundance in the HSQC spectrum. The S/G ratio in LRF was higher since the methoxyl group in S unit was removed by the acidic environment and leading to high G content. | ||
| Fig. 3 HSQC spectrum of main structures in the LRF: (A) aromatic area, (B) side chain area. The assignments of the 13C–1H cross signals were showed in Table S1.† Lignin substructure: (S) syringyl unit; (G) guaiacyl unit; (G′) oxidized guaiacyl unit; (H) p-hydroxyphenyl unit; (PCA) p-coumarated unit; (I) cinnamyl alcohol ending groups; (A′) acylated β-O-4 linkage. | ||
In the side chain areas (Fig. 3B), methoxy group had an apparent signal for the C–H correlation at δC/δH 56.0/3.72; the cinnamyl alcohol end groups showed Cγ–Hγ correlation at δC/δH 61.4/4.05; signals represented the Cγ–Hγ in γ-acylated β-O-4 linkage showed correlation at δC/δH 62.7/42.8. Based on the HSQC, signal corresponding to β-O-4 linkages were not observed in LRF while acylated β-O-4 linkages were detected. The breakdown of interlinkages could be caused by the acidolysis and ethanolysis. Similarly, Hallac et al. reported the decreasing β-O-4 linkages in B. davii after ethanol pre-treatment, which was caused by the homolytic breakage of β-O-4 linkages.30 Besides, El Hage et al. showed that organosolv pre-treatment would lead to a cleavage of β-O-4 linkages during the Miscanthus pre-treatment.31 In our study, most β-O-4 linkages and C–C linkages were severely broken down during the combined process of DASE and LHPAD which essentially was the acidolysis plus ethanolysis.
The HSQC signals in LRF from the associated carbohydrate (β-D-xylopyranoside, β-D-glucopyranoside etc.) could not be detected, which agreed with chemical composition analysis in Table 1 that LRF was pure. Assignments of 13C–1H correlation signals in the HSQC spectrum of the LRF were showed in Table S1.†
3.4 GPC analysis of LRF
The molecular weight of LRF was showed in Table 3 and Fig. S3.† The value was much lower than those obtained by other methods considerably. Lignin isolated from sugarcane bagasse by the phosphoric acid steam explosion and organosolv extraction had much higher molecular weight (Mw = 3731 g mol−1 with ethanol extraction and Mw = 3176 g mol−1 with dioxane extraction). In this study, acidolysis was used to cleave the aryl–ether bonds in heterogeneous system and steam explosion enhanced the process, which leading to the low molecular weight of LRF. In addition, ethanol was the most effective solvent and reactant for the degradation of lignin.32 Ethanol hydrolysis of corncob lignin could contribute to the cleavage of α-aryl ester bonds and β-aryl ester bonds. GPC results well tallied with HSQC spectrum that most linkages in LRC was cleaved by the combined process.3.5 XRD analysis of UN, SER and CRF
X-ray diffraction (XRD) was frequently used to analyse the cellulose structure and crystallinity. Fig. 4 showed the XRD spectrum of the UC, SER and CRF. There were two main peaks at 16.4° and 22.5° in the XRD spectrum of UC, which is the typical sign of cellulose I.33 However, in SER and CRF samples an additional small shoulder peak emerged at 21.8° besides the two main peaks at 16.4° and 22.5°. The emergence of small shoulder peak at 21.8° indicated the expansion of the lattice,34 which was caused by the expansion of the cellulose matrix structure and the removal of hemicellulose during the process of steaming explosion.35 The small peak at 34.5° represented the quarter length of one cellobiose unit along the fibre direction. In general, the characteristics of XRD spectrum in SER and CRF were not changed significantly in comparison to that in UC (cellulose I), although they preferred to forming cellulose II, in well agreement with the data of FTIR analysis. The crystal index (CrI) of UC, SER, CRF were 26.45%, 37.33% and 45.02%, respectively. The increased CrI after DASE and LHPAD should arise from the removal of amorphous hemicellulose.3.6 Effect of structural changes on the enzymatic hydrolysis
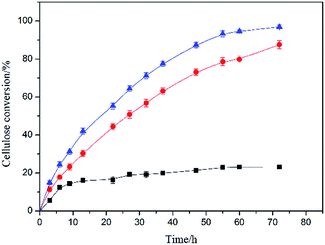
The cellulose conversion of untreated corncob, SER and CRF were 22.8%, 86.5% and 95.7% respectively (Fig. 5), indicating that the removal of hemicellulose and could facilitate the enzymatic hydrolysis of cellulose. However, the cellulose enzymatic hydrolysis rate was unsatisfactory and this was speculated that the cellulose structure was incompletely destroyed and small partial lignin in the CRF hindered the cellulose accessibility to cellulase.36 In the combined process, xylose, the precursor of xylitol and also a fine chemical, was obtained by degradation of hemicellulose with steaming explosion.14 Subsequently, the organosolv lignin was prepared from the SER by ethanol delignification, which was characterize with higher purity, yield and low-molecule-weight (Mw = 683 g mol−1, Fig. S3†). It had applications in petroleum feedstock, aromatic platform chemicals and functional fine chemicals.37 The similar process was used to extract lignin from corn stalk of which yield was close to our results, but the molecular weight of corn stalk lignin was much higher.38 It indicated that the lignin obtained from different biomass by the similar process had different characteristics and this process had potential to be applied on other types of biomass. In addition, three advantages could benefit the industrial application of the combined process: (1) trace amounts of H2SO4 for hemicellulose degradation, (2) recyclable and renewable ethanol for organosolv lignin preparation, (3) complete utilization of the components in lignocellulose of corncob. Consequently, the proposal process was environment friendly and industrially feasible, and it could be applied to biofuel production in the future.4. Conclusions
A cleaning, effective and economic process for lignin preparation from corncob was developed. In our work, diluted acid steam explosion (DASE) plus liquid hot pressured alcohol delignification (LHPAD) was applied on the production of lignin from corncob. DASE played an important role which could degrade the hemicellulose into xylose and remove it from the lignocellulose by wash. In addition, proper DASE give more accessible room for the next LHPAD, resulting in the high yield (41%) and high purity (94%). In addition, the cellulose rich fraction (LRF) after DASE and LHPAD was easy for enzymatic hydrolysis and the results showed that glucose conversion was close to 100%.Abbreviations
| UC | Untreated corncob |
| SER | Steam explosion residue |
| CRF | Cellulose rich fraction |
| LFR | Lignin rich fraction |
| DASE | Diluted acid steam explosion |
| LHPAD | Liquid hot pressured alcohol delignification |
Acknowledgements
We are indebted to the National High-tech Research and Development Program (2012AA022303, 2014AA021906), the Fundamental Research Funds for the Central Universities (YS1407) and State Grid Science and Technology Program (SGECS56-2014) for the generous financial supports.References
- http://www.stats.gov.cn/.
- D. Zhang, J. Liu and B. Li, Sustainability, 2014, 6, 5322 CrossRef PubMed.
- A. Brandt, M. J. Ray, T. Q. To and D. J. Leak, Green Chem., 2011, 13, 2489 RSC.
- R. Wahlström, S. Rovio and A. Suurnäkki, RSC Adv., 2012, 2, 4472 RSC.
- C. Mancera, F. Ferrando, J. Salvadó and N. E. El Mansouri, Biomass Bioenergy, 2011, 35, 2072 CrossRef CAS PubMed.
- H. Zhu, L. Wang, Y. Chen, G. Li, H. Li, Y. Tang and P. Wan, RSC Adv., 2014, 4, 29917 RSC.
- M. F. Patrick, P. Champagne, M. F. Cunningham and R. A. Whitney, Bioresour. Technol., 2010, 101, 8915 CrossRef PubMed.
- W. O. S. Doherty, P. Mousavioun and C. M. Fellows, Ind. Crops Prod., 2011, 33, 259 CrossRef CAS PubMed.
- R. E. Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrahi and A. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632 CrossRef PubMed.
- L. R. Lynd, E. Larson, N. Greene, M. Laser, J. Sheehan, B. E. Dale, S. McLaughlin and M. Wang, Biofuels, Bioprod. Biorefin., 2009, 3, 113 CrossRef CAS PubMed.
- S. N. Sun, X. F. Cao and X. M. Zhang, Bioresour. Technol., 2014, 163, 377 CrossRef CAS PubMed.
- W. D. S. Ramos, T. Poznyak, I. Chairez and I. Córdova, J. Hazard. Mater., 2009, 169, 428 CrossRef PubMed.
- D. Fu, G. Mazza and Y. Tamaki, J. Agric. Food Chem., 2010, 58, 2915 CrossRef CAS PubMed.
- J. Kim, E. Shin, I. Eom, K. Won, Y. H. Kim, D. Choi, I. Choi and J. W. Choi, Bioresour. Technol., 2011, 102, 9020–9025 CrossRef CAS PubMed.
- Z. Liu, P. Fatehi, M. S. Jahan and Y. Ni, Bioresour. Technol., 2011, 102, 1264 CrossRef CAS PubMed.
- N. S. Çetin and N. Özmen, Int. J. Adhes. Adhes., 2002, 22, 477 CrossRef.
- A. A. Pereira, G. F. Martins, P. A. Antunes, R. Conrrado, D. Pasquini, A. E. Job, A. A. S. Curvelo, M. Ferreira, A. Riul Jr and C. J. L. Constantino, Langmuir, 2007, 23, 6652 CrossRef CAS PubMed.
- G. Vázquez, C. Rodríguez-Bona, S. Freire, J. González-Álvarez and G. Antorrena, Bioresour. Technol., 1999, 70, 209 CrossRef.
- H. J. Zhang, X. G. Fan, X. L. Qiu, Q. X. Zhang, W. Y. Wang, S. X. Li, L. H. Deng, M. A. G. Koffas, D. S Wei and Q. P. Yuan, Bioprocess Biosyst. Eng., 2014, 37, 2425 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Croker. Determination of Structural Carbohydrates and Lignin in Biomass, NREL, 2008 Search PubMed.
- X. Fan, G. Cheng, H. Zhang, M. Li, S. Wang and Q. Yuan, Carbohydr. Polym., 2014, 114, 21 CrossRef CAS PubMed.
- X. J. Pan, J. F. Kadla, K. Ehara, N. Gilkes and J. N. Saddler, J. Agric. Food Chem., 2006, 54, 5806 CrossRef CAS PubMed.
- W. H. Chen, C. C. Tsai, C. F. Lin, P. Y. Tsai and W. S. Hwang, Bioresour. Technol., 2013, 128, 297 CrossRef CAS PubMed.
- G. Hu, C. Cateto, Y. Pu, R. Semuel and A. J. Raguaskas, Energy Fuels, 2012, 26, 740 CrossRef CAS.
- J. Zeng, Z. Tong, L. Wang, J. Y. Zhu and L. Ingram, Bioresour. Technol., 2014, 154, 274 CrossRef CAS PubMed.
- Y. Pu, N. Jiang and A. J. Ragauskas, J. Wood Chem. Technol., 2007, 27, 23 CrossRef CAS PubMed.
- R. E. Hage, N. Brosse, P. Sanigrahi and A. Ragauskas, Polym. Degrad. Stab., 2010, 95, 997 CrossRef PubMed.
- F. Carrillo, X. Colom, J. J. Suñol and J. Saurina, Eur. Polym. J., 2004, 40, 2229 CrossRef CAS PubMed.
- J. Bian, F. Peng, X. P. Peng, X. Xiao, P. Peng, F. Xu and R. C. Sun, Carbohydr. Polym., 2014, 100, 211 CrossRef CAS PubMed.
- B. B. Hallac, Y. Pu and A. J. Ragauskas, Energy Fuels, 2010, 24, 2723–2732 CrossRef CAS.
- R. E. Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrapi and A. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632 CrossRef PubMed.
- R. Ma, W. Hao, X. Ma, Y. Tian and Y. Li, Angew. Chem., Int. Ed., 2014, 53, 7310 CrossRef CAS PubMed.
- R. Kumar, G. Mago, V. Balan and C. E. Wyman, Bioresour. Technol., 2009, 100, 3948 CrossRef CAS PubMed.
- G. Cheng, P. Varanasi, R. Arora, V. Stavila, B. A. Simmons, M. S. Kent and S. Singh, J. Phys. Chem. B, 2012, 116, 10049 CrossRef CAS PubMed.
- J. Singh, M. Suhag and A. Dhaka, Carbohydr. Polym., 2015, 117, 624 CrossRef CAS PubMed.
- Z. Zhu, N. Sathitsuksanoh, T. Vinzant, D. J. Schell, J. D. McMillan and Y. P. Zhang, Biotechnol. Bioeng., 2009, 103, 715 CrossRef CAS PubMed.
- P. Gallezot, Catal. Today, 2007, 121, 76 CrossRef CAS PubMed.
- G. Wang and H. Chen, Sep. Purif. Technol., 2013, 120, 402 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12452b |
| This journal is © The Royal Society of Chemistry 2015 |