Sol–gel based simonkolleite nanopetals with SnO2 nanoparticles in graphite-like amorphous carbon as an efficient and reusable photocatalyst†
Abstract
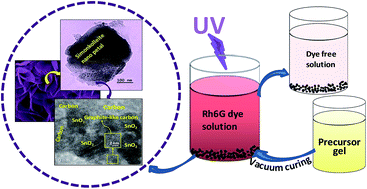
We report a new sol–gel nanocomposite (STC) having simonkolleite nanopetals (SC) and quasi-spherical tin oxide (SO) nanoparticles embedded in graphite-like amorphous carbon (C) as an efficient and reusable photocatalyst for the degradation of rhodamine 6G dye under UV (254 nm) illumination. The STC was synthesized using vacuum curing (450 °C) of precursor gel derived from a sol (Zn : Sn, 2 : 1) in 2-methoxyethanol with acetylacetone. The presence of tetragonal SO well decorated on rhombohedral SC forming nanoheterostructures in the carbon matrix was identified by X-ray diffraction, micro-Raman and X-ray photoelectron spectroscopy and electron microscopes (field emission scanning electron and transmission electron) studies. Carbon content and thermal weight loss behaviour in STC were studied by carbon determinator and thermogravimetry. The nanocomposite showed high photocatalytic activity (10−5 M dye solution degraded completely in 32 min). Reusability test of the photocatalyst exhibited about 95% of dye degradation after five successive recycles. In addition to accelerating photo-induced charge carrier separation and electron transport in the nanoheterostructures as revealed from electrochemical impedance spectroscopy response of the UV-exposed nanocomposite, an active role of the carbon at an optimum content (∼18%) was found for generating high BET specific surface area (∼143 m2 g−1). This simple synthesis strategy could open a new avenue to the development of sol–gel nanocomposites as efficient and reusable photocatalysts from various simonkolleite-based metal oxide semiconductors embedded in graphite-like amorphous carbon.

 Please wait while we load your content...
Please wait while we load your content...