Electrodeposition of a 3D hierarchical porous flower-like cobalt–MWCNT nanocomposite electrode for non-enzymatic glucose sensing
S. Premlatha,
P. Sivasakthi and
G. N. K. Ramesh Bapu*
CSIR-Central Electrochemical Research Institute, Electroplating and Metal Finishing Technology Division, Karaikudi-630006, Tamilnadu, India. E-mail: bapu2657@yahoo.com
First published on 21st August 2015
Abstract
In this work, pure cobalt and a cobalt–MWCNT nanocomposite were electrodeposited on a mild steel substrate using an acetate bath. The surface morphology, elemental composition and the preferred orientation of pure cobalt and the cobalt–MWCNT nanocomposite were characterized using SEM, EDAX and XRD analysis. The SEM images of the Co–MWCNT composite exhibited as nanoflakes which are interconnected to form a 3D hierarchical porous flower-like morphology. MWCNTs are well incorporated into the matrix. XRD also confirmed the composite formation and the presence of CNTs. Cyclic voltammetry and chronoamperometric techniques were employed to characterize the electrocatalytic activity of the modified electrode towards glucose detection. The results revealed that the proposed sensing material synergistically acts as an electrocatalyst for the electrooxidation of glucose with enhanced sensitivity and a lower detection limit. The fast current response signals, wide linear range and higher selectivity make the cobalt–MWCNT nanocomposite electrode a favorable candidate for practical glucose detection applications.
1. Introduction
Diabetes mellitus, the disease known for insulin deficiency has always been a public health problem. The tremendous increase in the diabetic population still attracts much attention to glucose sensing and the periodic monitoring of glucose is essential for the diagnosis of diabetes.1–3 Glucose detection is more significant in clinical diagnosis, food industry and biotechnology. A large number of enzymatic electrodes have been employed as glucose sensors since the discovery of the first glucose oxidase electrode in the 1960s.4–8 Enzymatic sensors have been commercialized owing to their sensitivity and selectivity. Though these electrodes have a higher electrocatalytic activity, enzymatic sensors suffer from elaborated enzyme immobilization processes, instability, pH and the high cost of the enzymes.9–11 Hence, there is a need to develop sensors which are fast and accurate and can pave the way for non-enzymatic glucose sensors. Electrochemical non-enzymatic sensors have attracted intense interest due to their high sensitivity, good reproducibility and low cost. Various approaches such as hydrothermal,12,13 sol–gel,14 electrospinning,5,15 and electrophoretic deposition16 methods have been applied to prepare sensor materials. However, these methodologies need sophisticated equipment and involve toxic chemicals. In addition to that, glucose oxidation on conventional drop casted electrodes hinders the practical use of the material because of its sluggish reaction kinetics. The higher potential needed for glucose determination consequently decreases the sensitivity.6 On the other hand, electrodeposition is a facile technique used to synthesize nano materials due to its cost effectiveness, environmental friendliness and ease of fabrication.17,18 Nowadays, transition metals,19 metal oxides,8,20 alloys21 and spinels5 are used for glucose sensing. Of these, transition metals have gained considerable attention for constructing practical glucose monitoring systems due to their availability and low cost. Particularly, cobalt and its oxides are well known for their electrocatalytic activity towards glucose oxidation owing to their higher catalytic activities, stability and multi valencies in alkaline media.18,22,23 Recently carbon nanotubes (CNTs) have been extensively utilized in electrochemical sensors because of their unique properties such as high surface to volume ratio, good electrical conductivity and stability. Moreover, CNTs present in nanocomposites can furnish conductivity for faster electron transport along with electroactive sites for spatial diffusion.24–27 Composite materials synthesized by adding highly conductive carbon nanotubes to metals and metal oxides can expect an enhanced performance owing to the synergistic effect between the two components rather than just one.28,29 Furthermore, nanoparticle incorporated composites always have enhanced physical and mechanical properties. Fenghua Su et al. prepared Co–MWCNTs composite coatings using different electrodeposition techniques and concluded that the composites have a greater hardness and corrosion resistance.30 Susumu Arai et al. synthesized Co–MWCNTs using a composite plating method and investigated its field emission properties.31 Cobalt based composites have been widely investigated for their tribological properties. Mahdavi et al. reported the characteristics of electrodeposited Co–TiO2 composite coatings.32 Although the electrocatalytic behaviour of Co ions in alkaline solutions has been well studied, the effect of incorporating nanoparticles into a Co matrix by electrodeposition and using the thus formed Co composite electrodes as non-enzymatic glucose sensors has not been studied. To the best of the authors’ knowledge, there are no reports on electrodeposited cobalt–MWCNT nanocomposite modified electrodes for glucose detection.In this work, an electrodeposition technique is employed to synthesize a Co–MWCNT composite electrode for detecting glucose selectively and in the presence of interferences such as urea, dopamine, ascorbic acid and NaCl as non-enzymatic sensors.
2. Experimental
2.1 Materials
All reagents were of analytical grade and were used ‘as received’ without further purification. MWCNTs were purchased from Tokyo chemical industry, Japan. Cobalt(II) acetate and boric acid were purchased from Merck, India. Standard solutions of glucose were freshly prepared prior to each experiment using distilled water. Ascorbic acid and dopamine were obtained from Alfa Aesar. Urea and KCl were supplied from Otto Inc. Mumbai, India.2.2 Preparation of the cobalt–MWCNT nanocomposite electrode
Mild steel plates of 7 cm × 1 cm × 0.5 mm were mechanically polished with grade 4–6 emery papers and then degreased with trichloroethylene. The unexposed area was masked with stop-off lacquer to acquire the required area for deposits. Further, it was subjected to alkaline electro-cleaning followed by acid pickling in 5% HCl solution. After that, the substrate was washed in running water and dipped into electrolyte containing 100 g L−1 cobalt(II) acetate, 40 g L−1 boric acid and the required amount of MWCNTs with a particle size of 10–20 nm (diam.). Before each experiment, the electrolyte was stirred continuously for 8 hours to blend the MWCNTs with the plating solution to get a uniform suspension. Fig. 1 shows and confirms the dispersion level of MWCNTs in the composite electrolyte. As can be seen, the particles are uniformly suspended in the composite electrolyte. Initially the electrocodeposition was performed by varying the concentration of MWCNTs from 1 g L−1 to 4 g L−1, the current density ranging from 2 A dm−2 to 4 A dm−2, the temperature from 30–50 °C and the pH from 2 to 4. By judging the quality and adherent nature of the deposit, the deposition parameters were optimized under which further co-deposition was performed using 2 g L−1 MWCNTs at the optimized conditions 4 A dm−2, 30 °C and pH 4 for 15 minutes using a two electrode system in which graphite plate acted as an anode and pre-treated mild steel as a cathode. The deposited film, of about 10 μm thickness was washed with distilled water and dried in air. 0.5 cm2 geometrical areas of the electrodeposits were used for glucose determination.2.3 Electrochemical characterization
The surface morphology and the elemental composition were analyzed using scanning electron microscopy and an energy dispersive X-ray spectrometer (Hitachi, model 3000H). The preferred orientation was evaluated by XPERT PRO X-ray diffractometry (XRD) using CuKα radiation with a wavelength of 1.5406 Å. The scanning range was between 20° and 90° with a step size of 0.04°.The electrochemical experiments for glucose sensing were carried out in a conventional three electrode set-up containing deposited cobalt and the cobalt–MWCNT nanocomposite as the working electrodes, platinum as the counter electrode and Ag/AgCl as the reference electrode using an SP-150 Bio-logic potentiostat (France). Cyclic voltammetry and chronoamperometry were employed to study the electrooxidation of glucose in 0.1 M NaOH aqueous solution.
3. Results and discussion
3.1 Characterization of the cobalt–MWCNT nano composite electrode
SEM images of pure cobalt and cobalt–MWCNTs are shown in Fig. 2. In the case of pure cobalt, the surface morphology (Fig. 2a) seems to be of cobalt nanoflakes that are uniformly distributed all over the substrate. As can be seen in Fig. 2b, the cobalt–MWCNT nanocomposite also appears as nanoflakes which are interconnected to form a 3D hierarchical flower-like porous structure. This unique morphology exists because the MWCNTs added into the bath act as a surface modifier and make the aggregated nanoflakes form a flower-like morphology, which is in agreement with the cited literature.33 | ||
| Fig. 2 SEM images of (a) pure cobalt (b) Co–MWCNT nanocomposite (c) single flower-like morphology of Co–MWCNTs and (d) FESEM image of Co–MWCNTs. | ||
Fig. 2c shows single hierarchical architecture revealed by a growth mechanism which originates from the core. The MWCNTs are entangled and well embedded on the layers of cobalt which is clearly visible at 100k magnification in the FESEM images shown in Fig. 2d. The possible mechanism for the flower-like structure is elucidated as the strong physical adsorbing tendency of the multi walled carbon nanotubes34 that are easily adsorbed onto the electrode surface providing active sites for the nucleation and growth of cobalt nuclei. Fig. 3 shows the backscattered SEM images of pure cobalt and cobalt–MWCNTs which clearly revealed the distribution of MWCNTs in the composite coatings.
The chemical compositions of pure cobalt and cobalt–MWCNT nanocomposite electrodeposits are shown in Fig. 4a and b. Pure cobalt thin films comprise cobalt, and oxide while the cobalt–MWCNT thin film contained cobalt, carbon and oxide with the wt% of 67, 30, and 3, respectively. The EDAX spectra analysis of the composite deposits confirmed 30 wt% distribution of MWCNTs in the composite coatings.
3.2 XRD analysis
For the Co–MWCNT nanocomposite, the preferred orientation changed from the (100) plane to the (110) plane and its intensity was increased compared to that of pure cobalt suggesting a reduction in grain size and the existence of a preferable crystal structure.35 Moreover, the existence of a new peak at (101) indicated a ‘hcp’ texture. The incorporation of MWCNTs not only changed the orientation but also decreased the grain size.30 The peak located at the 2θ values of 31.6751 and 44.6725 could be assigned to the crystal structure of Co3O4 which corresponds to the (220) and (400) planes of the standard JCPDS 42-1467.1,33 The peak remaining at 2θ = 28.4278 could be indexed to the CNTs (Fig. 5).3.3 Co–MWCNT electrodeposit as a glucose sensor
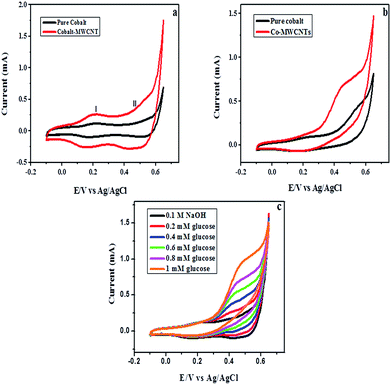
Fig. 6a shows cyclic voltammograms for pure cobalt and Co–MWCNT nanocomposite electrodes in the absence of glucose in 0.1 M NaOH solution at a scan rate of 50 mV s−1. In both cases, two well defined redox pairs were obtained. Peak I at 0.2 V could be attributed to the conversion of Co2+ to Co3+ and peak II at 0.5 V is assigned to the oxidation of Co3+ to Co4+ which is in good agreement with the cited literature.36–39 The oxidation current density for Co–MWCNTs (0.45 mA cm−2) is higher than that of pure cobalt (0.16 mA cm−2) indicating the synergistic effect of cobalt and MWCNTs which is highly useful for electrochemical sensing applications. Fig. 6b shows the CVs for the oxidation of 1 mM glucose added into the 0.1 M NaOH solution at a scan rate of 20 mV s−1. In the case of cobalt, the onset potential of glucose oxidation starts at E = 0.4 V whereas for the Co–MWCNT modified electrode the onset potential is E = 0.27 V, ascribed to the enhanced performance due to the MWCNTs. Upon addition of 1 mM glucose into 0.1 M NaOH, the oxidation peak (II) current increases from 0.36 mA cm−2 to 0.66 mA cm−2 and the potential shift towards the negative direction reveals that glucose is oxidized to gluconolactone by CoO2/CoOOH redox couple.20,39 As expected, addition of MWCNTs into the cobalt matrix increases the oxidation current, lowering the overpotential for glucose compared with that of pure cobalt. Fig. 6c represents the cyclic voltammograms of the Co–MWCNT nanocomposite electrode for different concentrations of glucose. It can be seen that the oxidation current increases with an increase in glucose concentration.| Co3O4 + OH− + H2O ↔ 3CoOOH + e− |
| CoOOH + OH ↔ CoO2 + H2O + e− |
| 2CoO2 + C6H12O6 → 2CoOOH + C6H10O6 |
The results revealed that the potential shift and anodic current increment is due to the synergistic effect of cobalt and MWCNTs.
3.4 Amperometric detection of glucose
All the amperometric experiments were carried out at 0.43 V vs. Ag/AgCl which is optimized from the CV experiments. Fig. 7a shows the amperometric response of the Co–MWCNT nanocomposite electrode in the presence of glucose in a stirred solution of 0.1 M NaOH. For each addition of glucose, the current increased steeply followed by the attainment of a steady state current which represents the constant increment in oxidation current upon different concentrations of glucose. The calibration curve for glucose detection was obtained by plotting the current values with glucose concentration. At an applied potential of 0.43 V, the sensor displays two linear detection ranges, one in the low concentration and another in the high concentration range. Two linear ranges have also been reported in the literature in glucose sensing with the reasoning that increasing the glucose concentration blocks the mass transfer that affects the diffusion of fresh glucose molecules nearer to the electrode surface. At concentrations higher than 0.1 mM, the electrochemical response is flattened.40 Consequently, two discrete linear ranges were obtained for the Co–MWCNT modified electrode. As shown in Fig. 7b the calibration curve is linear for glucose concentrations ranging from 5 μM to 100 μM with another linear behavior for high concentrations of glucose ranging from 200 μM to 3600 μM (Fig. 7c). At higher concentrations of glucose, the amperometric curve reached its saturation point, hence, some amperometric current response was denied due to the adsorbed intermediates blocking the active catalytic sites.41 Furthermore, the limit of detection for lower concentrations of glucose was calculated as 0.009 μM whereas for the higher concentration range, the detection limit was estimated to be 0.3 μM at a signal to noise ratio of 3 with correlation coefficients of 0.992 and 0.989. The sensitivity was calculated from the slope of the calibration curve and it was found to be 727 μA mM−1 cm−2 and 37.05 μA mM−1 cm−2. The steady state response time for each addition of glucose was reached within 3 s revealing rapid electron transport on the electrode surface. In particular, while increasing the glucose concentration, the anodic current increased but the non-linearity above 3600 μM may be due to the limit generated from the oxidation rate or the saturation of a number of catalytic sites for oxidation.5 The analytical performance of the proposed sensor was compared with those of other glucose sensors reported in the literature and these are presented in Table 1. As can be seen, the analytical parameters of this glucose sensor are better than the other sensors reported in the literature. Particularly, the limit of detection for lower concentrations is 9 nM which is superior to the other modified electrodes reported in the literature.18,25,28 The enhanced analytic performance is because of the three dimensional hierarchical porous structure of the Co–MWCNT nanocomposite electrode.| Electrode materials | Applied potential (V) | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | Detection limit (μM) | References |
|---|---|---|---|---|---|
| Co–MWCNT nanocomposite | 0.43 | 727.37 | 0.005–0.1, 0.2–3.6 | 0.009, 0.3 | This work |
| CoOOH nanosheets | 0.5 | 967 | Up to 0.5 | 10.9 | 38 |
| Co3O4–RGO | 0.5 | 1145.2 | 0.1–0.9 | 10 | 1 |
| Co3O4 nanofibers | 0.5 | 36.3 | Up to 2 | 0.97 | 39 |
| CoOOH nanosheet arrays | 0.5 | 526.8 | Up to 1.109 | 1.37 | 22 |
| 3D graphene–cobalt oxide | 0.5 | 3390 | Up to 0.08 | 0.025 | 36 |
| MWCNT–CoTsPc | 0.3 | 0.1225 | 0.01–6.34 | 0.14 | 25 |
| CoOx·nH2O–MWCNT | 0.55 | 162.8 | Up to 4.5 | 2 | 18 |
| CoOx NPs/ERGO | 0.6 | 79.3 | 0.01–0.55 | 2 | 29 |
3.5 Interference test
In order to establish the selectivity of the Co–MWCNT nanocomposite electrode for glucose detection, commonly interfering species like ascorbic acid, dopamine, and urea were added along with glucose. The physiological levels of these interferents are much less than glucose. In addition to that, chloride ion poisoning is an issue associated with non enzymatic glucose sensors.29,34 Hence, 0.1 M of NaCl was added in order to investigate the effect of chloride ions. 0.1 mM concentration of glucose was added initially and the oxidation current increased, whereas, for the addition of 0.1 M concentration of inteferents, no obvious current response was seen, as shown in Fig. 8.The current response of the inteferents is very low compared to glucose, which is evidence for the selective detection of glucose molecules. Hence, this modified composite electrode can be used for the sensing of glucose even in the presence of other interferents.
3.6 Reproducibility and stability
In an attempt to evaluate the reproducibility of the Co–MWCNT nanocomposite electrode, five different electrodes were prepared under similar conditions and the amperometric response was recorded upon injection of 45 μM of glucose. A relative standard deviation (RSD) of 3.03% was obtained indicating good reproducibility. The sensor was stored in a desiccator in order to establish long term usage. The long term stability of the proposed sensor was evaluated every five days for the addition of 60 μM of glucose. 97.3% of the current was retained after 45 days which revealed its excellent stability.3.7 Real sample analysis
To determine the practicability of the sensor, the Co–MWCNT nanocomposite electrode was used to analyze the glucose in blood serum. The source of the human blood serum used in our experiments was obtained from volunteers in our institute, i.e., CSIR-CECRI Health Centre, Karaikudi clinical setting. All amperometric experiments involving the use of blood serum were performed in compliance with the relevant laws and our institutional guidelines, and the institutional CSIR-CECRI Health Care committee has approved the experiments. As per guide lines, the consent of blood serum sources have been informed and obtained. Fig. 9 represents the amperometric response of 0.1 mM and 0.2 mM of standard glucose solution followed by a human blood serum sample. 100 μl of blood serum was added into 10 ml of 0.1 M NaOH and the amperometric response was recorded. The amount of glucose present in the serum was estimated as 6 mM by comparing the known current value of standard glucose concentration. The clinically reported value is 5.8 mM which is close to the estimated values using this sensor material indicating the practical use of this sensor. | ||
| Fig. 9 Amperometric current response of the Co–MWCNT nano composite in the presence of various concentrations of glucose followed by blood serum in 0.1 M NaOH. | ||
4. Conclusions
In this study, a versatile and cost-effective electrodeposition technique is adopted to synthesize cobalt and cobalt–MWCNT nanocomposite electrodes and the fabricated material is successfully demonstrated as a non-enzymatic glucose sensor. Hierarchical porous structure and the incorporation of CNTs into the Co matrix synergistically enhanced the electron transfer resulting in a lower detection limit of 0.009 μM with a high sensitivity of 727 μA mM−1 cm−2 and good selectivity towards glucose detection. The proposed sensor is highly stable for over a month. Hence, it can provide a great pathway for the real time application of monitoring glucose.Acknowledgements
The authors thank the Director, CSIR-CECRI for his kind permission to publish the paper. One of the authors (SP) gratefully acknowledges the University Grants Commission, New Delhi, India (Grant No. F1-17.1/2012–13/RGNF-SC-TAM-34623) for financial support of this research work under the Rajiv Gandhi National Fellowship scheme.Notes and references
- M. Li, C. Han, Y. Zhang, X. Bo and L. Guo, Anal. Chim. Acta, 2014, 1–11 CrossRef PubMed
.
- J. Wang, in Electrochemical Sensors, Biosensors and their Biomedical Applications, 2008, pp. 57–69 Search PubMed
.
- K. Dhara, T. Ramachandran, B. G. Nair and T. G. Satheesh Babu, J. Electroanal. Chem., 2015, 743, 1–9 CrossRef CAS PubMed
.
- N. Hui, W. Wang, G. Xu and X. Luo, J. Mater. Chem. B, 2015, 3, 556–561 RSC
.
- Y. Zhang, L. Luo, Z. Zhang, Y. Ding, S. Liu, D. Deng, H. Zhao and Y. Chen, J. Mater. Chem. B, 2014, 2, 529–535 RSC
.
- A. Sun, J. Zheng and Q. Sheng, Electrochim. Acta, 2012, 65, 64–69 CrossRef CAS PubMed
.
- S. Liu, B. Yu and T. Zhang, Electrochim. Acta, 2013, 102, 104–107 CrossRef CAS PubMed
.
- G. Wang, X. Lu, T. Zhai, Y. Ling, H. Wang, Y. Tong and Y. Li, Nanoscale, 2012, 4, 3123–3127 RSC
.
- R. Thota and V. Ganesh, Analyst, 2014, 139, 4661–4672 RSC
.
- C. K. Tan, K. P. Loh and T. T. L. John, Analyst, 2008, 133, 448–451 RSC
.
- P. Zhang, L. Zhang, G. Zhao and F. Feng, Microchim. Acta, 2011, 176, 411–417 CrossRef
.
- C. Karuppiah, S. Palanisamy, S.-M. Chen, V. Veeramani and P. Periakaruppan, Sens. Actuators, B, 2014, 196, 450–456 CrossRef CAS PubMed
.
- S. Soyoon, A. Ramadoss, B. Saravanakumar and S. J. Kim, J. Electroanal. Chem., 2014, 717–718, 90–95 CrossRef CAS PubMed
.
- A. H. Jayatissa, K. Guo, A. C. Jayasuriya and T. Gupta, Mater. Sci. Eng., B, 2007, 144, 69–72 CrossRef CAS PubMed
.
- C. Zhou, L. Xu, J. Song, R. Xing, S. Xu, D. Liu and H. Song, Sci. Rep., 2014, 4, 7382 CrossRef CAS PubMed
.
- P. Subramanian, J. Niedziolka-Jonsson, A. Lesniewski, Q. Wang, M. Li, R. Boukherroub and S. Szunerits, J. Mater. Chem. A, 2014, 2, 5525–5533 CAS
.
- F. Su, C. Liu, Q. Zuo, P. Huang and M. Miao, Mater. Chem. Phys., 2013, 139, 663–673 CrossRef CAS PubMed
.
- J. Yang, W. Zhang and S. Gunasekaran, Electrochim. Acta, 2011, 56, 5538–5544 CrossRef CAS PubMed
.
- M.-J. Song, S.-K. Lee, J.-H. Kim and D.-S. Lim, J. Electrochem. Soc., 2013, 160, B43–B46 CrossRef CAS PubMed
.
- C.-W. Kung, C.-Y. Lin, Y.-H. Lai, R. Vittal and K.-C. Ho, Biosens. Bioelectron., 2011, 27, 125–131 CrossRef CAS PubMed
.
- J. Wang, Z. Wang, D. Zhao and C. Xu, Anal. Chim. Acta, 2014, 832, 34–43 CrossRef CAS PubMed
.
- L. Zhang, C. Yang, G. Zhao, J. Mu and Y. Wang, Sens. Actuators, B, 2015, 210, 190–196 CrossRef CAS PubMed
.
- L. Wang, X. Lu, Y. Ye, L. Sun and Y. Song, Electrochim. Acta, 2013, 114, 484–493 CrossRef CAS PubMed
.
- X. Kang, Z. Mai, X. Zou, P. Cai and J. Mo, Anal. Biochem., 2007, 363, 143–150 CrossRef CAS PubMed
.
- R. Devasenathipathy, C. Karuppiah, S.-M. Chen, S. Palanisamy, B.-S. Lou, M. A. Ali and F. M. a. Al-Hemaid, RSC Adv., 2015, 5, 26762–26768 RSC
.
- Y. Lin, F. Lu, Y. Tu and Z. Ren, Nano Lett., 2004, 191–195 CrossRef CAS
.
- M. Yang, J. Jiang, Y. Yang, X. Chen, G. Shen and R. Yu, Biosens. Bioelectron., 2006, 21, 1791–1797 CrossRef CAS PubMed
.
- M. Shamsipur, M. Najafi and M. R. M. Hosseini, Bioelectrochemistry, 2010, 77, 120–124 CrossRef CAS PubMed
.
- S. J. Li, J. M. Du, J. Chen, N. N. Mao, M. J. Zhang and H. Pang, J. Solid State Electrochem., 2014, 18, 1049–1056 CrossRef CAS
.
- F. Su, C. Liu, J. Guo and P. Huang, Surf. Coat. Technol., 2013, 217, 94–104 CrossRef CAS PubMed
.
- S. Arai and K. Miyagawa, Appl. Surf. Sci., 2013, 280, 957–961 CrossRef CAS PubMed
.
- S. Mahdavi and S. R. Allahkaram, Surf. Coat. Technol., 2013, 232, 198–203 CrossRef CAS PubMed
.
- H. Heli, I. Eskandari, N. Sattarahmady and a. a. Moosavi-Movahedi, Electrochim. Acta, 2012, 77, 294–301 CrossRef CAS PubMed
.
- C. Zhang, G. Wang, M. Liu, Y. Feng, Z. Zhang and B. Fang, Electrochim. Acta, 2010, 55, 2835–2840 CrossRef CAS PubMed
.
- P. Liu, Z. Hu, Y. Liu, M. Yao and Q. Zhang, J. Alloys Compd., 2015, 622, 805–811 CrossRef CAS PubMed
.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed
.
- I. G. Casella and M. R. Guascito, Electrochim. Acta, 1999, 45, 1113–1120 CrossRef CAS
.
- K. K. Lee, P. Y. Loh, C. H. Sow and W. S. Chin, Electrochem. Commun., 2012, 20, 128–132 CrossRef CAS PubMed
.
- Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang and Y. Lei, Biosens. Bioelectron., 2010, 26, 542–548 CrossRef CAS PubMed
.
- Y. He and J. Zheng, Anal. Methods, 2013, 5, 767–772 RSC
.
- L. Han, D. P. Yeng and A. Liu, Biosens. Bioelectron., 2015, 63, 145–152 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |