Validated stability-indicating RP-LC method for the determination of pemirolast potassium investigation of kinetic behavior
Abstract
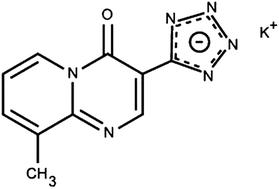
The stability of the anti-asthmatic drug pemirolast potassium was investigated under different stress conditions, including hydrolysis (acid and alkali), heat, light and oxidation as recommended by the ICH guidelines. The degradation process was found to take place only under alkaline and oxidative conditions. A stability-indicating RP-LC method was developed and validated for the determination of pemirolast potassium in the presence of its degradation products and process-related impurities. The chromatographic analysis was achieved on an Eclipse®, XDB-C18 (150 mm × 4.6 mm, 5 μm) column, under isocratic elution by a mixture of water: methanol: glacial acetic acid, pH 3.5 50 : 50 : 0.3 (v/v/v), as a mobile phase, delivered at 1.0 ml min−1 at 258 nm. Method validation was demonstrated to be selective, accurate and precise with good linearity over the concentration range of 1–10 μg ml−1 with limits of detection and quantification of 21 and 69 ng ml−1, respectively. Robustness against small modifications of pH and the percentage of the aqueous mobile phase was ascertained. The developed method was successfully applied for the analysis of pemirolast potassium in commercial eye drops and tablets; therefore it is highly suitable for routine analysis in QC labs. Moreover this method was utilized to investigate the kinetics of the alkaline degradation of pemirolast potassium, determine the order of the degradation rate constant, calculate the rate constant, half-life time and estimate the drug shelf-life (expiry date), and the activation energy of the degradation process.

 Please wait while we load your content...
Please wait while we load your content...