Size dependent electronic band structures of β- and γ-graphyne nanotubes
Baotao Kang *a,
Jong Hun Moonb and
Jin Yong Lee*b
*a,
Jong Hun Moonb and
Jin Yong Lee*b
aSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong 250022, People's Republic of China. E-mail: kangbaotao@hotmail.com
bDepartment of Chemistry, Sungkyunkwan University, Suwon, 440-746, Republic of Korea. E-mail: jinylee@skku.edu
First published on 16th September 2015
Abstract
In the present paper, density functional theory calculations have been implemented by using Dmol3 to study the electronic band structures of β-graphyne nanotubes (βGyNTs) and γ-graphyne nanotubes (γGyNTs). Our results found different GyNTs show diverse electronic band structures. All βGyNTs have quite small band gaps without any correlation with tube size. Meanwhile, γGyNTs, no matter zigzag or armchair, exhibit semiconductor characteristics with oscillatory band gap ranging from 0.48 eV to 1.20 eV. Furthermore, based on the variation of band gap, both zigzag and armchair γGyNTs can be divided into two subgroups: 2m and 2m + 1 where n is a positive integer, following the order of 2m + 1 > 2m.
Introduction
During the last two decades, carbon-based materials including fullerenes,1,2 carbon nanotubes (CNTs)3,4 and graphene,5 have received enormous amounts of attention from theorists and experimentalists. Especially, CNTs constituted by only sp2-like carbons could be either metallic or semiconducting, and they have been intensively exploited in electronic devices.6,7 Recently a new carbon allotrope by inserting acetylenic linkage (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) into graphene, named graphyne, has been receiving growing attention. The coexistence of sp and sp2 carbons grants graphyne outstanding characteristics.8–12 These unique properties could allow graphyne to have some potential applications.13–15 The presence of acetylenic linkages allows graphyne to exist in several different two-dimensional structures, such as α-, β-, and γ-graphyne.9–11 Since CNTs can be considered as seamless cylinders of graphene sheets, we can also build graphyne nanotubes (GyNTs) via the same approach. Recently, graphdiyne nanotubes (GDyNTs) has been successfully synthesized and exhibited high-performance field emission properties,16 which gives a possibility for realization of single-walled graphyne nanotubes (GyNTs).
C–) into graphene, named graphyne, has been receiving growing attention. The coexistence of sp and sp2 carbons grants graphyne outstanding characteristics.8–12 These unique properties could allow graphyne to have some potential applications.13–15 The presence of acetylenic linkages allows graphyne to exist in several different two-dimensional structures, such as α-, β-, and γ-graphyne.9–11 Since CNTs can be considered as seamless cylinders of graphene sheets, we can also build graphyne nanotubes (GyNTs) via the same approach. Recently, graphdiyne nanotubes (GDyNTs) has been successfully synthesized and exhibited high-performance field emission properties,16 which gives a possibility for realization of single-walled graphyne nanotubes (GyNTs).
Comparing with CNTs, GyNTs have not received much attention though it deserved.17,18 Computational studies have been carried out to investigate the electronic band structure via tight-binding (TB) method.19,20 However, there are two very import parameters ignored in TB method, σ–π hybridization effect21 and the coexistence of sp and sp2 hybridized carbon. Such a weakness of TB has been exposed by recent studies on CNTs. Theoretical and experimental approaches have proved that zigzag (n, 0) CNTs are typical semiconductor when n ≠ 3m,22,23 while zigzag (n, 0) CNTs when n = 3m were predicted to be metallic via the TB method.24,25 Afterwards, recent research based on DFT calculations disclosed a small energy band gap,26,27 which has been experimentally confirmed.28 The (n, 0) SWCNTs with n = 3m showed semiconductor property with band gap of 0.080 ± 0.005 eV for (9, 0), 0.042 ± 0.004 eV for (12, 0) and 0.029 ± 0.004 eV for (15, 0) SWCNT. As such different electronic band structure of αGyNTs by TB method compared with our calculations based on density functional theory (DFT) was revealed.29 To our knowledge, DFT calculations have not been systematically implemented for β-graphyne nanotubes (βGyNTs) and γ-graphyne nanotubes (γGyNTs), which motivates us to undertake the present study. From the calculated electronic band structure, we found that βGyNTs and γGyNTs possess different electronic structures from ordinary CNTs and αGyNTs. βGyNTs have small band gaps without correlation with tube size, while γGyNTs are typical semiconductor but with different oscillatory band gap behavior compared to the CNTs.
Computational method
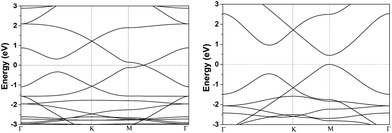
In this study, DFT-D calculations were carried out by DMol3 module30 in Material Studio 5.5. In earlier paper, different DFT method including PWC functional31 belonging to LDA, PW91,31 BLYP32 and PBE functionals33,34 belonging to GGA, were implemented to investigate the band structures of αGyNTs and gave nearly the same results. Thus, in the present paper PBE method was utilized with the consideration of dispersive interaction correction (PBE-D). Such PBE-D method has been intensively applied to study carbon related materials.35,36 Moreover, there is no spin restriction imposed during calculations. For all calculations, 20 Å vacuum space was imposed to avoid interlayer interactions. All electron treatment was performed and a double numerical plus polarization (DNP) basis set was used. The convergence tolerance of energy was 10−5 hartree, and the maximum allowed force and displacement were 0.002 hartree per Å and 0.005 Å, respectively. The unit cell of β- and γ-graphyne was fully optimized with 2D hexagonal symmetry with k-point meshes of 7 × 7 × 1, and the lattice constant (a) was computed to be 9.527 and 6.923 Å, respectively. As noted in Fig. 1, the band structure of β-graphyne has a zero band gap, while that of γ-graphyne has a band gap (Eg) of 0.47 eV at M high symmetric point. Dispersive interactions are important in electronic structure calculations of carbon materials such as graphene.37 Herein we compared the Eg obtained with and without dispersion correction. The Eg without dispersion correction was reported to be 0.42 eV,20 which was further verified by our result of 0.43 eV by merely PBE method. Since pure DFT often underestimates band gap, dispersion correction could relieve such a problem.Based on the optimal unit cell of β- and γ-graphyne, the corresponding zigzag and armchair nanotubes were built by rolling up film along different orientation. The structure of each nanotube was first fully optimized with Brillouin zone k-point meshes of 1 × 1 × 4. Then the k-point meshes was increased to 2 × 2 × 50, which should be large enough to obtain reliable electronic band structure (50 is the maximum value allowed by Dmol3). For each tube, the cohesive energy (Ecoh) was calculated as follows:
| Ecoh = Etotal/NC − EC |
Results and discussion
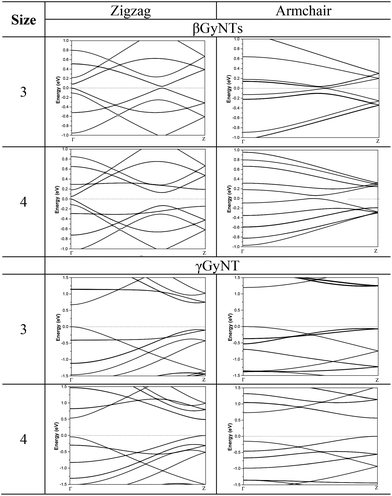
As we did in earlier study,29 the chirality of tubes was characterized by applying nomenclature (n, m). As shown in Fig. 2, the chirality of βGyNTs and γGyNTs is slightly different from that of αGyNTs possessing hexagonal carbon ring. To maintain consistency with αGyNTs, zigzag and armchair were still used to name the chirality of βGyNTs and γGyNTs. Following such notation, (n, 0) and (n, n) stand for zigzag and armchair nanotubes, respectively. These tubes were denoted by Nz-Z-β (γ) GyNTs and Na-A-β (γ) GyNTs, where Nz and Na represent the tube size of zigzag and armchair nanotubes, respectively. Fig. 2 shows the optimized structures of βGyNTs and γGyNTs with Nz and Na being 4 for instance. The relationship between cohesive energy (Ecoh) and tube size (Nz or Na) was pictured in Fig. 3. The cohesive energy for βGyNTs and γGyNTs monotonically decreased as the tube size increases implying gradual reduction in surface strain. For both βGyNTs and γGyNTs, the cohesive energy of armchair was always smaller than that of zigzag when Nz = Na because A-GyNTs has larger diameter. The cohesive energy of βGyNTs and γGyNTs gradually converged to −7.11 and −7.32 eV per atom since surface strain tends to decrease. Those values are larger than that of αGyNTs with −7.02 eV per atom. Such values are comparable to that of fullerene with −7.29 eV per atom38 that makes the single walled GyNT to be realized in near future.Then the corresponding electronic band structure of βGyNT with Nz = 3 and 4 were plotted in Fig. 4. For 3-Z-βGyNT, one can see that the valence band maximum (VBM) reaches to the Fermi level (EF) and the conduction band minimum (CBM) locates above EF, inducing a direct band gap (Eg) of 0.03 eV between Γ and Z point. Meanwhile, the location of such direct band gap moves to the top of Γ point for 4-Z-βGyNT whose Eg was 0.05 eV. Further analysis confirmed the dependence of band gap's location upon the parity of Nz. To elucidate the size dependence of band gap, the variation of Eg as a function of Nz was shown in Fig. 5 and no correlation between Eg and tube size was found for Z-βGyNTs.
The band structure of Z-βGyNTs is distinctly diverse from zigzag CNTs and αGyNTs. It was determined that zigzag (n, 0) CNTs are semiconductor and show an oscillatory band gap dependence on tube size (n). The band gap follows the rank order: 3m − 1 > 3m + 1 > 3m.22,23,28 Recently, we carried out reliable DFT calculations to investigate the electronic structures of αGyNTs and found Z-αGyNTs possess qualitatively similar band gap behavior.29 All Z-αGyNTs are semiconductors and exhibit strong dependence on tube size. Nz-Z-αGyNTs can be classified into three families according to band gap variation: Nz = 3m − 1, 3m, and 3m + 1 resulting in the following rank order of band gap magnitudes: 3m − 1 > 3m + 1 > 3m.
For the band structure of A-βGyNTs, as displayed in Fig. 4, the VBM reaches to EF, and the CBM locates above EF, inducing a direct band gap between Γ and Z point. The Eg of A-βGyNTs was calculated to be about 0.01 eV except for the cases of Nz = 4 and 9. The Eg of 4- and 9-A-βGyNT were 0.06 eV and 0.11 eV, respectively. Considering DFT usually underestimates band gap, A-βGyNTs could be concluded as semiconductor. Similar to Z-βGyNTs, A-βGyNTs do not show a size dependence of band gap as noted in Fig. 5 and have different electronic band structure compared to armchair CNTs and A-αGyNTs. Armchair CNTs had been experimentally determined to be metallic.28,39 Furthermore, A-αGyNTs were concluded to be semiconductors when tube size is small and showed downward trend on tube size, then becomes metallic as the tube size increases.
The band structure of γGyNTs showed that the VBM reaches to EF and the CBM locates above EF, thus resulting in direct band gaps. Similar to Z-βGyNTs, the band gap location of γGyNTs was found to be related with the parity of tube size. When Nz or Na was odd, the direct band gap located at Γ point, while that of even Nz or Na located at Z point. Furthermore, all γGyNTs were semiconductors with moderate Eg ranging from 0.48 eV to 1.20 eV as pictured in Fig. 5. Moreover, it was further revealed that the band gap of Z-γGyNTs exhibited oscillatory and size dependent behavior, which is similar to zigzag CNTs and αGyNTs. However, differently from zigzag CNTs and αGyNTs, Z-γGyNTs could be classified into two families with Nz = 2m and 2m + 1 (n is a positive integer) following the order of band gap magnitudes: 2m + 1 > 2m according to band gap variation. Additionally, armchair CNTs, αGyNTs and βGyNTs are either semiconductor with very small band gap or metal, while A-γGyNTs are typical semiconductor with moderate band gaps. Similar to Z-γGyNTs, A-γGyNTs can also be divided into two subgroups with Na = 2m and 2m + 1 following the order of band gap magnitudes: 2m + 1 > 2m. The oscillatory behavior of the band gap of γGyNTs was also observed from DFTB study.17 But one point should be emphasized that the band gap of A-γGyNTs in present paper is always larger than that of Z-γGyNTs when Na = Nz, while it is overturned by DFTB method.17 Such disharmony may originate from the inadequacy of tight-binding-link treatment in DFTB method. It would be elusive until more expensive calculations like GW method40 or experiments report the band gaps of γGyNTs in the future. In addition, the band gaps of both Z-γGyNTs and A-γGyNTs gradually decreased as tube size increased, and were predicted to converge to that value of γ-graphyne film.
Conclusions
Our DFT calculation showed different GyNTs have diverse electronic band structures. αGyNTs possess qualitatively similar band gap behaviour to ordinary CNTs. Z-αGyNTs were disclosed as semiconductors with band gap which is strongly dependent on tube size. Nz-Z-αGyNTs can be classified into three families: Nz = 3m − 1, 3m, and 3m + 1 resulting in the following rank order of band gap magnitudes: 3m − 1 > 3m + 1 > 3m. Meanwhile, A-αGyNTs are semiconductors with small tube size then becomes metallic as the tube size increases.29 However, βGyNTs appeared to be atypical among CNTs and GyNTs. All βGyNTs have quite small band gaps but without any correlation with tube size. For γGyNTs, no matter zigzag or armchair, semiconductor character was clearly observed with moderate band gap of 0.48–1.20 eV. Both Z- and A-γGyNTs can be divided into two subgroups: 2m and 2m + 1 where n is positive integer, following the order of band gap magnitudes: 2n +1 > 2n. Their band gaps decreased as tube size increases, and were predicted to converge to the value of 2D γ-graphyne. Moreover, the band gap of A-γGyNTs is always larger than that of Z-γGyNTs. According to the electronic properties, γGyNTs would be a potential candidate as semiconductors with controllable band gap by tuning the tube size.Acknowledgements
This work was supported by National Research Foundation (NRF) grants funded by the Korean government (MEST) (2007-0056343). We acknowledge the financial support from the Ministry of Education, Science and Technology, subjected to the project EDISON (Education-research Integration through Simulation On the Net, Grant No.: 2012M3C1A6035359).Notes and references
- D. M. Guldi, B. M. Illescas, C. M. Atienza, M. Wielopolskia and N. Martin, Chem. Soc. Rev., 2009, 38, 1587–1597 RSC
.
- T. Braun, A. P. Schubert and R. N. Kostoff, Chem. Rev., 2000, 100, 23–27 CrossRef CAS PubMed
.
- C. Wang, K. Takei, T. Takahashi and A. Javey, Chem. Soc. Rev., 2013, 42, 2592–2609 RSC
.
- P. M. Ajayan, Chem. Rev., 1999, 99, 1787–1799 CrossRef CAS PubMed
.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed
.
- M. S. Dresselhaus, G. Dresselhaus, J. C. Charlier and E. Hernandez, Philos. Trans. R. Soc., A, 2004, 362, 2065–2098 CrossRef CAS PubMed
.
- M. F. L. de Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed
.
- Y. Li, L. Xu, H. Liu and Y. Li, Chem. Soc. Rev., 2014, 43, 2572–2586 RSC
.
- J. Kang, J. Li, F. Wu, S. S. Li and J. B. Xia, J. Phys. Chem. C, 2011, 115, 20466–20470 CAS
.
- S. W. Cranford and M. J. Buehler, Carbon, 2011, 49, 4111–4121 CrossRef CAS PubMed
.
- Y. Y. Zhang, Q. X. Pei and C. M. Wang, Appl. Phys. Lett., 2012, 101, 081909 CrossRef PubMed
.
- B. Kang, H. Liu and J. Y. Lee, Phys. Chem. Chem. Phys., 2014, 16, 974–980 RSC
.
- H. Zhang, M. Zhao, X. He, Z. Wang, X. Zhang and X. Liu, J. Phys. Chem. C, 2011, 115, 8845–8850 CAS
.
- C. Li, J. Li, F. Wu, S. S. Li, J. B. Xia and L. W. Wang, J. Phys. Chem. C, 2011, 115, 23221–23225 CAS
.
- B. Kang and J. Y. Lee, J. Phys. Chem. C, 2014, 118, 12035–12040 CAS
.
- G. X. Li, Y. L. Li, X. M. Qian, H. B. Liu, H. W. Lin, N. Chen and Y. J. Li, J. Phys. Chem. C, 2011, 115, 2611–2615 CAS
.
- X. M. Wang and S. S. Lu, J. Phys. Chem. C, 2013, 117, 19740–19745 CAS
.
- Y. S. Wang, P. F. Yuan, M. Li, W. F. Jiang, Q. Sun and Y. Jia, J. Solid State Chem., 2013, 197, 323–328 CrossRef CAS PubMed
.
- V. R. Coluci, S. F. Braga, S. B. Legoas, D. S. Galvao and R. H. Baughman, Phys. Rev. B, 2003, 68, 035430 CrossRef
.
- V. R. Coluci, S. F. Braga, S. B. Legoas, D. S. Galvao and R. H. Baughman, Nanotechnology, 2004, 15, S142–S149 CrossRef CAS
.
- X. Blase, L. X. Benedict, E. L. Shirley and S. G. Louie, Phys. Rev. Lett., 1994, 72, 1878–1881 CrossRef CAS
.
- H. W. Wang, B. C. Wang, W. H. Chen and M. Hayashi, J. Phys. Chem. A, 2008, 112, 1783–1790 CrossRef CAS PubMed
.
- R. B. Weisman and S. M. Bachilo, Nano Lett., 2003, 3, 1235–1238 CrossRef CAS
.
- J. W. Mintmire, B. I. Dunlap and C. T. White, Phys. Rev. Lett., 1992, 68, 631–634 CrossRef CAS
.
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 1804–1811 CrossRef CAS
.
- Y. Matsuda, J. Tahir-Kheli and W. A. Goddard, J. Phys. Chem. Lett., 2010, 1, 2946–2950 CrossRef CAS
.
- T. Ito, K. Nishidate, M. Baba and M. Hasegawa, Surf. Sci., 2002, 514, 222–226 CrossRef CAS
.
- M. Ouyang, J. L. Huang, C. L. Cheung and C. M. Lieber, Science, 2001, 292, 702–705 CrossRef CAS PubMed
.
- B. Kang and J. Y. Lee, Carbon, 2015, 84, 246–253 CrossRef CAS PubMed
.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS PubMed
.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef
.
- A. D. Becke, J. Chem. Phys., 1988, 88, 2547–2553 CrossRef CAS PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS
.
- J. P. Perdew, K. Burke and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 16533–16539 CrossRef CAS
.
- J. Ma, A. Michaelides, D. Alfe, L. Schimka, G. Kresse and E. G. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 033402 CrossRef
.
- W. M. Sun, Y. X. Bu and Y. X. Wang, J. Phys. Chem. C, 2011, 115, 3220–3228 CAS
.
- M. Alonso, T. Woller, F. J. Martin-Martinez, J. Contreras-Garcia, P. Geerlings and F. De Proft, Chem.–Eur. J., 2014, 20, 4931–4941 CrossRef CAS PubMed
.
- X. Hua, T. Cagin, J. Che and W. A. Goddard, Nanotechnology, 2000, 11, 85–88 CrossRef CAS
.
- J. W. G. Wildoer, L. C. Venema, A. G. Rinzler, R. E. Smalley and C. Dekker, Nature, 1998, 391, 59–62 CrossRef CAS
.
- G. Onida, L. Reining and A. Rubio, Rev. Mod. Phys., 2002, 74, 601–659 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |