Synthesis and characterization of acrylic acid-2-hydroxyethyl methacrylate IPN hydrogels
Abstract
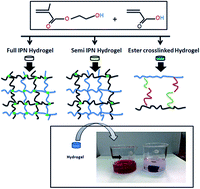
Interpenetrating polymer networks (both full and semi) and ester crosslinked hydrogels have been synthesized from acrylic acid and 2-hydroxyethyl methacrylate by using various monomer feed compositions. No crosslinkers have been used in case of ester crosslinked gel, instead the neighboring polymer chains are crosslinked by esterification reaction, which is established by FTIR and 13C NMR spectroscopy. Swelling of the hydrogels has been found to be strongly dependent on the pH, temperature and ionic strength of the medium. The rheological properties of the hydrogels are found to be dependent on the mode of crosslinking and composition of the gel (only in the case of ester crosslinked gel) as well. The flexibility of the gel is also dependent on the method of preparation of the hydrogel. Rheological study in normal force sweep mode reveals the high shape and size regain ability of the gel. The cryo SEM images show the porous structure of the gels. The hydrogels, especially ester crosslinked gels, have been found quite effective in the separation of cationic dyes and heavy metal ions (copper and iron) from their corresponding aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...