Synthesis and characterization of novel biobased benzoxazines from cardbisphenol and the properties of their polymers
Shengfang Li*ab and
Shilin Yanc
aSchool of Chemistry and Chemical Engineering, Hubei Polytechnic University, Huangshi 435003, PR China. E-mail: lishengf_@163.com
bHubei Key Laboratory of Mine Environmental Pollution Control and Remediation, Hubei Polytechnic University, Huangshi 435003, PR China
cWuhan University of Technology, Whuhan 430070, PR China
First published on 13th July 2015
Abstract
Two types of novel biobased benzoxazine monomers (named as CBB and CBF, respectively) from cardbisphenol were synthesized by a solventless method. The chemical structure of the monomers was confirmed by FTIR, 1H NMR and 13C NMR spectra, etc. FTIR and DSC showed that CBB and CBF were thermally initiated and polymerized via ring-opening polymerization. As a result, the thin polybenzoxazine (named as PCBB and PCBF, respectively) films can be obtained. Dynamic mechanical analysis (DMA) indicated that the glass transition temperatures (Tg) of PCBB and PCBF were 94 °C and 121 °C, respectively. Thermogravimetric analysis (TGA) demonstrated that the thermal stability of PCBF was better that of PCBB. Both PCBB and PCBF had low humidity absorption values.
1. Introduction
Nowadays, polybenzoxazines as a novel developed high performance phenolic resin have gained considerable attention over the past decade because they exhibit excellent properties, such as nearly zero shrinkage upon curing, high glass transition temperature, low water absorption, limited byproduct release upon curing and good flame retardancy.1–3 Moreover, the benzoxazine chemistry synthesis offers a wide range of molecular design flexibility on benzoxazine monomers, which can be synthesized from a phenolic derivate, formaldehyde and a primary amine compound via the Mannich reaction.4–8 These fascinating characteristics make polybenzoxazine resins a promising candidate in different application areas, for example, electronics, space-flight and aerospace.9Currently, synthesis of biobased benzoxazine from renewable resources i.e., eugenol, vanilline and urushiol has become a rapidly growing area, as these materials could potentially replace or partially replace environmentally and energy unfavorable petroleum-based materials.10–15 Cardanol is an industrial yellow oil extracted from cashew shell liquid (CNSL), which is also a promising renewable phenol resource. So far, several research groups have reported synthesis of various benzoxazines (i.e., cardanol/ammonia, cardanol/aniline, cardanol/furfurylamine, cardanol/allylamine, cardanol/ethanolamine, cardanol/aromatic or aliphatic diamine) from cardanol to replace part of the petroleum-based phenolic compound.16–22 However, almost all of these literatures only focused on synthesizing biobased benzoxazines using monophenol as starting materials. Few literatures reported biobased benzoxazines using bisphenol derivated from renewable resources as starting materials. Very recently, in our previous study, the cardanol–furfural phenolic resins were also used to prepare oligomeric benzoxazine precursors and the highly thermally stable polybenzoxazines could be obtained.23 As we all know, cardbisphenol is a aliphatic-bridged bisphenol derivated from cardanol, which has been used to prepare Mannich base.24 However, to our best knowledge, biobased benzoxazines from cardbisphenol have never been reported up till now.
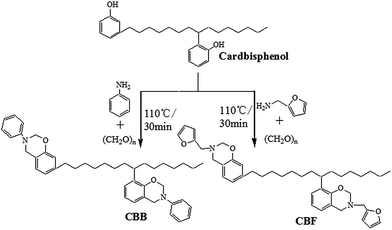
In this paper, we attempt to synthesize two types of novel biobased benzoxazine monomers (CBB and CBF) using the cardbisphenol, amine, furfurylamine and paraformaldehyde as starting raw materials as shown in Fig. 1. The thermally activated polymerization of CBB and CBF as well as the properties of the corresponding polybenzoxazines (PCBB and PCBF) was discussed in details.
2. Experimental
2.1. Materials
Cardanol was purchased from Shanghai Cashew Chemical Polymer Company (China). Phenol, paraformaldehyde (95%), furfurylamine, amine and chloroform were purchased from Shanghai First Reagent Company (China). Cardbisphenol prepared from distilled cardanol and phenol according to the modified method in the previous publication.25 The yield was 65%. FTIR spectra (KBr), cm−1: 685, 770, 825, 1170, 1485, 1510, 1610, 3350. 1H NMR (CDCl3, 300 MHz, δ ppm): 7.24–6.65 (m, 8H), 2.59–2.87 (m, H), 2.52 (t, 2H), 1.55–1.20 (m, 24H), 0.84 (s, 3H). All chemicals were AR grade and used without further purification.2.2. Measurements
FTIR spectrum was obtained on a PerkinElmer-2 spectrometer (KBr pellet). 1H (300 MHz) and 13C (100.5 MHz) nuclear magnetic resonance (NMR) spectra were obtained with a Bruker spectrometer with Fourier transform, with CDCl3 as a solvent, and TMS as an internal standard. Elemental analysis was performed with a VARIO EL III rapid elemental analyzer. DSC was measured with a heating rate of 10 °C min−1 under N2 atmosphere on Perkin-Elmer DSC6 apparatus. Dynamic scans of the samples were recorded. TGA were performed on a DuPont 2000 thermogravimetric analyzer. Cured samples were weighed in the sample pan and then heated in the TGA furnace at a heating rate of 5 °C min−1 under N2 atmosphere. The resulting thermograms were recorded. Scanning electron microscopy (SEM) of the fractured surfaces was performed using Hitachi ISI-SX-40 SEM. The fractured surfaces of the specimens were covered with gold vapor. The acceleration voltage was 20 kV. The Tg of the polybenzoxazine film was measured with a dynamic mechanical analyzer (DMA, Thermal Analysis DMA-Q800). The applied static force and dynamic force were 0.11 and 0.10 N, respectively. The heating rate was 5 °C min−1 and the frequency was 1 Hz. The peak temperature of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ plot was taken as Tg of the sample. Humidity absorption of cured samples were conditioned under vacuum at 90 °C for 20 h before being placed in air (79% and 29% relative humidity (RH)). All these experiments were conducted at room temperature. Then, the humidity absorption content (HA) of the cured samples was calculated according to formula (1) as follows:26
δ plot was taken as Tg of the sample. Humidity absorption of cured samples were conditioned under vacuum at 90 °C for 20 h before being placed in air (79% and 29% relative humidity (RH)). All these experiments were conducted at room temperature. Then, the humidity absorption content (HA) of the cured samples was calculated according to formula (1) as follows:26| HA(%) = (Wt − W0) × 100%/W0 | (1) |
2.3. Synthesis of biobased benzoxazine monomers from cardbisphenol
CBB was prepared by the previous reported solventless method.27 Namely, a mixture of cardbisphenol, aniline and paraformaldehyde with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was added into a flask and stirred at 110 °C for 30 min. The product was dissolved in dichloromethane. The resulting solution was filtered and washed with deionized water and the solvent was removed by rotary evaporation at reduced pressure. The product (CBB) was dried at reduced pressure and room temperature to provide a brown yellow powder. The yield was 85%. m.p.: 58 °C. Anal. C43H54O2N2; C 81.90%; H 8.57%; N 4.44%; found: C 82.21%; H 8.12%; N 4.03%. FTIR spectra (KBr), cm−1: 753, 807, 897, 960, 991, 1031, 1076, 1115, 1144, 1241, 1372, 1431, 1457, 1497, 1601, 2853, 2925, 3026. 1H NMR (CDCl3, 300 MHz, δ ppm): 7.25–6.63 (m, 9H), 5.34 (s, 4H), 4.60 (s, 4H), 2.50–2.49 (d, 2H), 1.96 (m, 4H), 1.55 (s, 2H), 1.28–1.25 (d, 20H), 0.87 (t, 3H). 13C NMR (CDCl3, δ ppm): 14, 22, 25, 27, 29, 31, 32, 34, 35, 48, 50, 79, 107, 108, 110, 115, 116, 119, 120, 121, 127, 129, 142, 152 and 153.
4 was added into a flask and stirred at 110 °C for 30 min. The product was dissolved in dichloromethane. The resulting solution was filtered and washed with deionized water and the solvent was removed by rotary evaporation at reduced pressure. The product (CBB) was dried at reduced pressure and room temperature to provide a brown yellow powder. The yield was 85%. m.p.: 58 °C. Anal. C43H54O2N2; C 81.90%; H 8.57%; N 4.44%; found: C 82.21%; H 8.12%; N 4.03%. FTIR spectra (KBr), cm−1: 753, 807, 897, 960, 991, 1031, 1076, 1115, 1144, 1241, 1372, 1431, 1457, 1497, 1601, 2853, 2925, 3026. 1H NMR (CDCl3, 300 MHz, δ ppm): 7.25–6.63 (m, 9H), 5.34 (s, 4H), 4.60 (s, 4H), 2.50–2.49 (d, 2H), 1.96 (m, 4H), 1.55 (s, 2H), 1.28–1.25 (d, 20H), 0.87 (t, 3H). 13C NMR (CDCl3, δ ppm): 14, 22, 25, 27, 29, 31, 32, 34, 35, 48, 50, 79, 107, 108, 110, 115, 116, 119, 120, 121, 127, 129, 142, 152 and 153.
CBF was also synthesized using cardbisphenol, furfurylamine and paraformaldehyde as raw materials by the same method. The yield was 84%. m.p.: 55 °C. Anal. C41H54O4N2; C 77.12%; H 8.46%; N 4.39%; found: C 77.63%; H 8.22%; N 4.12%. FTIR spectra (KBr), cm−1: 753, 809, 867, 885, 938, 968, 995, 1012, 1076, 1121, 1149, 1240, 1361, 1430, 1458, 1503, 1578, 2854, 2927. 1H NMR (CDCl3, 300 MHz, δ ppm): 7.41 (d, 2H), 7.35–6.64 (m, 6H), 6.33 (d, 2H), 6.14 (d, 2H), 5.19 (s, 4H), 4.89 (s, 4H), 4.76 (s, 4H), 2.52 (d, 4H), 1.95 (d, 4H), 1.58 (s, 18H), 1.21 (s, 2H), 0.85 (s, 3H). 13C NMR (CDCl3, δ ppm): 14, 22, 25, 27, 29, 31, 32, 34, 35, 36, 37, 40, 50, 79, 116, 117, 118, 120, 121, 126, 128, 129, 143, 148 and 154.
2.4. Preparation of biobased polybenzoxazines
The biobased polybenzoxazines were prepared by thermally polymerization according to the following method: about 40% by weight solution of CBB or CBF in tetrahydrofuran solution was placed over a glass plate. After most of the solvent was removed under ambient atmosphere at 60 °C, the glass plate was placed into a vacuum oven at 60 °C for 24 h to remove the residual solvent. After that, the vacuum oven was subjected to a step curing procedure as follows: 120 °C (2 h), 150 °C (2 h), 180 °C (2 h), 200 °C (2 h), 230 °C (2 h), respectively. Upon completion of the curing, the samples (named as PCBB or PCBF) were allowed to freely cool to room temperature and cut appropriate dimension to be applied for property evaluation.3. Results and discussion
3.1. Preparation and characterization of the biobased benzoxazine monomers
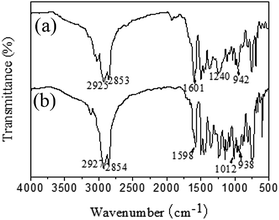
Cardbisphenol based benzoxazine monomers (CBB and CBF) were synthesized by the condensation reaction of cardbisphenol with paraformaldehyde and aniline or furfurylamine as shown in Fig. 1. The yield of CBB and CBF were 85% and 84%, respectively. Both of them were brown yellow powder at room temperature and showed a good solvency in common solvents.The structure of CBB and CBF was confirmed by FTIR, 1H NMR and 13C NMR spectral methods. Fig. 2 shows the FTIR spectra of CBB and CBF, respectively. The typical absorption band of benzoxazine ring for CBB appeared at 942 cm−1, whereas for CBF appeared at 938 cm−1. For both CBB and CBF, other characteristic absorption bands can be also observed at 1012–1121 cm−1 (symmetric stretching of C–O–C), about 1240 cm−1 (asymmetric stretching of C–O–C), about 1144 cm−1 (symmetric stretching vibrations of C–N–C) and about 1361 cm−1 (wagging of CH2). In addition, the absorption peaks at 1490–1601 cm−1 was assigned to the trisubstituted benzene groups. The characteristic absorption bands at about 2927 cm−1 (–CH2–) and about 2854 cm−1 (–CH3) were assigned to the aliphatic alkyl chain from cardbisphenol. Furthermore, the characteristic absorption peaks of furan rings in CBF was found at 1578, 968, and 753 cm−1. Another characteristic absorption peak of furan ring at 938 cm−1 overlapped with the absorption band of benzoxazine ring.23
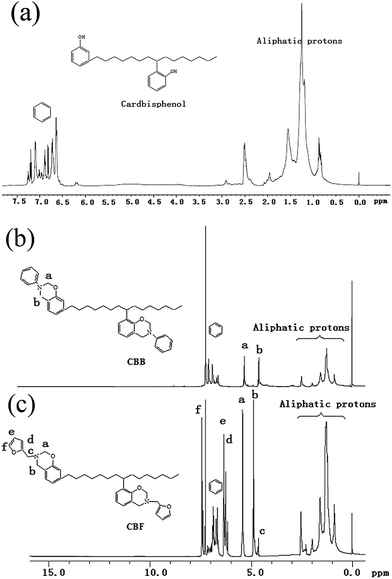
Fig. 3 shows the 1H NMR spectra of cardbisphenol, CBB and CBF, respectively. Comparing with Fig. 3a–c, it was very clear that both CBB and CBF had the same characteristic protons of aliphatic alkyl chain appeared at 2.52, 1.95, 1.58, 1.21 and 0.85 ppm as well as cardbisphenol, respectively. The aromatic ring protons appeared as multiplets between 6.63 and 7.35 ppm. Furthermore, for CBB, the characteristic protons of oxazine ring appeared at 5.34 and 4.60 ppm (–O–CH2–N– and Ar–CH2–N–), respectively (Fig. 3b). However, for CBF, the characteristic protons of oxazine ring appeared at 5.19 and 4.89 ppm (–O–CH2–N– and Ar–CH2–N–), respectively (Fig. 3c).1–3 In addition, for CBF, the protons of the furan ring gave peaks at 6.2, 6.3, and 7.4 ppm, while the methylene group connecting the oxazine ring and the furan ring resonated at 4.76 ppm.23,28
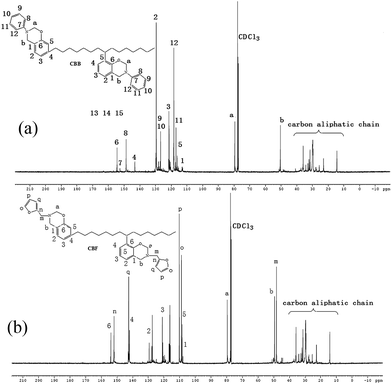
Fig. 4 shows the 13C NMR spectra of CBB and CBF, respectively. As shown in Fig. 4, the characteristic carbon resonances of the oxazine ring were found at 50 and 79 ppm for Ar–CH2–N and N–CH2–O, respectively. The peak at 48 and 152 ppm was attributed to the methylene group carbons connecting the oxazine ring and the furan ring. In addition, the peaks at 108, 110 and 142 ppm were assigned to the furan ring carbons.
3.2. Thermally activated polymerization of the biobased benzoxazine monomers
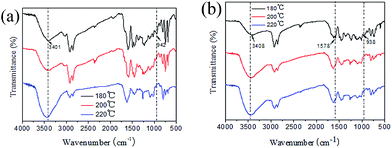
Thermally activated polymerization of CBB and CBF was investigated by FTIR and DSC methods. Fig. 5a and b show the FTIR spectra of CBB and CBF after curing at different temperature. Comparing with the FTIR spectra of monomers in Fig. 2, for both CBB and CBF, the intensity of characteristic peaks at 942 or 938 cm−1 assigned to benzoxazine ring decreased significantly to a considerable extent when the curing temperature was elevated from 180 °C to 220 °C. At the same time, the intensity of absorption peak at 3401 or 3408 cm−1 assigned to –OH increased markedly as the curing reaction advanced. All the results suggested that the ring opening reaction of oxazine ring had occurred in both CBB and CBF when the temperature was elevated gradually.3,5 Additionally, the absorption of furan ring at 1578 cm−1 became broad after polymerization, indicating the formation of disubstituted furan ring. Formation of disubstituted furan rings in polymerization indicated that furan ring participated in the polymerization reactions.28Fig. 6 shows the DSC thermograms of CBB and CBF with the results summarized in Table 1. The endothermic peaks centered around 55–58 °C were attributed to the melting point of the benzoxazine monomers. Then an exothermic behavior observed at high temperature region associated to the ring-opening polymerization of oxazine rings. The onset polymerization temperatures of CBB and CBF were 214 °C and 219 °C, respectively, which were comparable to the polymerization temperatures of other benzoxazine monomers.1–3 The exothermic maxima were observed at 242 °C and 253 °C for CBB and CBF, respectively. The temperature difference between melting point and onset of polymerization is often defined as the processing window. According to the calculation, it was found the processing window for CBB and CBF was 156 °C and 164 °C, respectively. This indicated both CBB and CBF had wide processing windows, which were beneficial to some resin transfer molding process.
| Sample | Tm (°C) | Tonset (°C) | Tmax (°C) | Tprocessing window (°C) | ΔH/J g−1 |
|---|---|---|---|---|---|
| CBB | 58 | 214 | 242 | 156 | 83.6 |
| CBF | 55 | 219 | 253 | 164 | 112.3 |
The reaction enthalpies of polymerizations of CBB and CBF were found as 83.6 and 112.3 J g−1, respectively. In other words, the exothermic enthalpy of CBF was much higher than that of CBB. The main reason may be that, for CBF, the additional reaction of furan ring in molecular backbone as stated above could increase the exothermic enthalpy.28
3.3. Thermal properties of the biobased polybenzoxazines
The thermal stability of the biobased polybenzoxazines was further studied by TGA (Fig. 7). The results are summarized in Table 2. Fig. 7 indicated that the thermal properties of two biobased polybenzoxazines at high temperature range (especially above 400 °C) were very similar. However, the thermal properties of two polybenzoxazines were obvious different when the temperature is in the range from 200 °C to 400 °C. From Table 2, it can be seen that the 5% and 10% weight loss temperature (T5 and T10) of PCBB were 304 °C and 348 °C, whereas T5 and T10 of PCBF were 356 °C and 374 °C, respectively. Moreover, the char yields of PCBB and PCBF at 800 °C were 21.74% and 22.50%, respectively. Obviously, the thermal stability of PCBF was superior to that of PCBB. This may be attributed to the fact that the additional reaction of furan ring in the molecular backbone of CBF could increase the crosslink density of the polybenzoxazine. Similar results were also reported previously.23,28| Sample | T5 (°C) | T10 (°C) | Char yield (800 °C) |
|---|---|---|---|
| PCBB | 304 | 348 | 21.74% |
| PCBF | 356 | 374 | 22.50% |
3.4. Film-forming abilities of the biobased polybenzoxazines
It is very known that the high brittleness and the difficulties in preparing thin films are mainly disadvantages associated with conventional bisphenol-A based polybenzoxazine.1 Thus, we attempted to prepare a thin polybenzoxazine film by thermally polymerization of the as-synthesized biobased benzoxazines to overcome the shortcomings of bisphenol-A based benzoxazine. As a result, for both CBB and CBF, we found a brown yellow and transparent thin polybenzoxazine film could be obtained easily according to a step curing process. There were no bubbles and cracks on the surface of polybenzoxazine films, exhibiting good film-forming abilities. In addition, these obtained polybenzoxazine films were flexible in some degree due to the introduction long aliphatic chain alkyl groups in cardbisphenol, which can overcome the brittleness brought by the highly rigid aromatic backbone in the conventional bisphenol-A based polybenzoxazine. They can be bent as shown Fig. 8. However, the flexibility of PCBF film reduced comparing with that of PCBB because of higher crosslink density resulted from the additional reaction of furan rings as stated above. | ||
| Fig. 8 Thin film photographs of the biobased polybenzoxazines before and after bending. (a) PCBB and (b) PCBF, respectively. | ||
The fractured surfaces of the obtained biobased polybenzoxazine films were further investigated by SEM. As a result, for both PCBB and PCBF, the fibrous patterns and plastic deformations could be observed. These fibrous patterns and plastic deformations can absorb energy upon impacting due to the introduction of the aliphatic alkyl chain of cardbisphenol, indicating a tough fractured surface (Fig. 9a–d).29
 | ||
| Fig. 9 SEM photographs of the fractured surfaces of the polybenzoxazines. (a) PCBB; (b) PCBB; (c) PCBF; (d) PCBF. The magnification was ×150 for (a and c); and ×1000 for (b and d), respectively. | ||
3.5. Mechanical properties of the biobased polybenzoxazines
DMA was performed to study the thermomechanical properties of PCBB and PCBF. As seen in Fig. 10, the storage modulus (E′) of PCBB and PCBF at room temperature were 7.25, 2.94 GPa, respectively. The E′ value for PCBF started to decrease at about 93 °C, whereas for PCBB at about 58 °C. Comparing the decreasing slope of the E′ value from glassy state to rubbery plateau, it was found that the decreasing slope of PCBF was much lower than that of PCBB. This demonstrated that PCBF had higher mechanic performance than that of PCBB.Tg was determined from the peak maximum of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curve. Fig. 11 shows the tan
δ curve. Fig. 11 shows the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves for the biobased polybenzoxazines. In general, a flexible polybenzoxazine shows a low Tg due to the poor thermal stability. Mele et al. reported that the polybenzoxazine prepared from cardanol, formaldehyde and ammonia exhibited a Tg of 36 °C.16 Patton et al. synthesized a series of aliphatic-bridged bisphenol-based benzoxazines by varying the length of the aliphatic-bridged bisphenol used in the monomer synthesis. They found that the Tgs of the resulting polybenzoxazines were in a range from 66 °C to 101 °C.30,31 Agag et al. reported that Tgs of the polybenzoxazines containing pendent aliphatic chains were in a range from 81 °C to 92 °C.32 Interestingly, the result of tan
δ curves for the biobased polybenzoxazines. In general, a flexible polybenzoxazine shows a low Tg due to the poor thermal stability. Mele et al. reported that the polybenzoxazine prepared from cardanol, formaldehyde and ammonia exhibited a Tg of 36 °C.16 Patton et al. synthesized a series of aliphatic-bridged bisphenol-based benzoxazines by varying the length of the aliphatic-bridged bisphenol used in the monomer synthesis. They found that the Tgs of the resulting polybenzoxazines were in a range from 66 °C to 101 °C.30,31 Agag et al. reported that Tgs of the polybenzoxazines containing pendent aliphatic chains were in a range from 81 °C to 92 °C.32 Interestingly, the result of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves showed that Tgs of PCBB and PCBF were 94 °C and 121 °C, respectively (Fig. 11). This meant that the Tgs of the present cardbisphenol based polybenzoxazines were relatively higher than those flexible cardanol based mono-functional polybenzoxazines, other flexible aliphatic-bridged bisphenol based polybenzoxazines or the flexible polybenzoxazines containing pendent aliphatic chains reported previously.16,30–32
δ curves showed that Tgs of PCBB and PCBF were 94 °C and 121 °C, respectively (Fig. 11). This meant that the Tgs of the present cardbisphenol based polybenzoxazines were relatively higher than those flexible cardanol based mono-functional polybenzoxazines, other flexible aliphatic-bridged bisphenol based polybenzoxazines or the flexible polybenzoxazines containing pendent aliphatic chains reported previously.16,30–32
In order to better understand the above phenomenon, crosslink density ρ(E′) can be estimated from the rubbery plateau storage modulus at Tg + 40 °C according to the theory of rubbery elasticity (eqn (2)) as follows:33–36
| ρ(E′) = E′/3ϕRT | (2) |
3.6. Humidity adsorption of the biobased polybenzoxazines
The humidity absorption of the biobased polybenzoxazines is shown in Fig. 12. As shown in Fig. 12a and b, although the values of HA increased slightly with an increase in time, both PCBB and PCBF exhibited low humidity absorption values at room temperature. For PCBF and PCBB, When RH = 29%, HA < 0.52%, 0.46%, respectively. Whereas when RH = 79%, HA < 0.77%, 0.52%, respectively. The low humidity absorption abilities of PCBB and PCBF were mainly attributed to the introduction of the long hydrophobic aliphatic chain alkyl groups in cardbisphenol. Moreover, PCBF showed lower humidity absorption ability than that of PCBB due to the higher crosslink density.4. Conclusions
In this paper, two types of biobased benzoxazine monomers (CBB and CBF) with good film-forming abilities have been successfully synthesized from cardbisphenol. The structures of the monomers were supported by FTIR, 1H NMR and 13C NMR spectra. The thin polybenzoxazine films can be obtained easily by ring-opening polymerization of corresponding biobased benzoxazine monomers. SEM of the fractured surface of the obtained biobased polybenzoxazine films indicated a tough fractured surface. Moreover, TGA and DMA showed that the thermal and mechanical properties of PCBF were higher than that of PCBB because that the additional reaction of furan rings in PCBF could increase the crosslink density of the cured sample. The HA values of two polybenzoxazines were very low at high or low relative humidity. This new class of biobased polybenzoxazines has a potential for use in thin films devices, membranes and coatings applications where improved flexibility and thermomechanical properties.Acknowledgements
The project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, Hubei provincial Science & Technology Department (2013CFB040), Hubei Polytechnic University (13xjz01A; 2012101), China.References
- N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344–1391 CrossRef CAS PubMed.
- R. S. Kumar, M. Ariraman and M. Alagar, RSC Adv., 2014, 4, 19127–19136 RSC.
- M. Zhang, Z. Tan, H. Ma, J. Qiu and C. Liu, RSC Adv., 2014, 4, 53505–53513 RSC.
- S. Li, W. Huang, X. Liu, X. Yu and W. Xiao, Polym. Adv. Technol., 2010, 21, 229–234 CrossRef CAS PubMed.
- S. Li and M. Tao, J. Therm. Anal. Calorim., 2013, 113, 633–639 CrossRef CAS.
- K. Sethuraman and M. Alagar, RSC Adv., 2015, 5, 9607–9617 RSC.
- M. G. Mohamed, C. H. Hsiao, F. Luo, L. Dai and S. W. Kuo, RSC Adv., 2015, 5, 45201–45212 RSC.
- J. Liu, C. Scott, S. Winroth, J. Maia and H. Ishida, RSC Adv., 2015, 5, 16785–16791 RSC.
- S. Rajesh Kumar, J. Dhanasekaran and S. Krishna Mohan, RSC Adv., 2015, 5, 3709–3719 RSC.
- G. Lligadas, A. Tϋzϋn, J. C. Ronda, M. Galià and V. Cádiz, Polym. Chem., 2014, 5, 6636–6644 RSC.
- P. Thirukumaran, A. Shakila and S. Muthusamy, RSC Adv., 2014, 4, 7959–7966 RSC.
- H. Xu, Z. Lu and G. Zhang, RSC Adv., 2012, 2, 2768–2772 RSC.
- H. Xu, W. Zhang, Z. Lu and G. Zhang, RSC Adv., 2013, 3, 3677–3682 RSC.
- N. K. Sini, J. Bijwe and I. K. Varma, J. Polym. Sci., Part A: Polym. Chem., 2013, 43, 5267–5282 Search PubMed.
- A. Van, K. Chiou and H. Ishida, Polymer, 2014, 55, 1443–1451 CrossRef CAS PubMed.
- E. Calò, A. Maffezzoli, G. Mele, F. Martina, S. E. Mazzetto, A. Tarzia and C. Stifani, Green Chem., 2007, 9, 754–759 RSC.
- B. Lochab, I. Varma and J. Bijwe, J. Therm. Anal. Calorim., 2010, 102, 769–774 CrossRef CAS.
- B. Lochab, I. Varma and J. Bijwe, J. Therm. Anal. Calorim., 2012, 107, 661–668 CrossRef CAS.
- B. S. Rao and A. Palanisamy, Prog. Org. Coat., 2012, 74, 427–434 CrossRef CAS PubMed.
- B. S. Rao and A. Palanisamy, React. Funct. Polym., 2011, 71, 148–154 CrossRef CAS PubMed.
- S. Li, T. Zou, L. Feng, X. Liu and M. Tao, J. Appl. Polym. Sci., 2013, 128, 4164–4171 CrossRef CAS PubMed.
- P. Thirukumaran, P. A. Shakila and M. Sarojadevi, ACS Sustainable Chem. Eng., 2014, 2, 2790–2801 CrossRef CAS.
- S. Li, S. Yan, J. Yu and B. Yu, J. Appl. Polym. Sci., 2011, 122, 2843–2848 CrossRef CAS PubMed.
- M. Ramasri, G. S. S. Rao, P. S. Sampathkumaran and M. M. Shirsalkar, J. Appl. Polym. Sci., 1990, 39, 1993–2004 CrossRef CAS PubMed.
- M. Ramascri, G. S. S. Rao, P. S. Sampathkumaran and M. M. Shirsalkar, Indian J. Chem., 1987, 26(B), 683–684 Search PubMed.
- M. Zhang, Z. Tan, S. Hu, J. Qiu and C. Liu, RSC Adv., 2014, 4, 44234–44243 RSC.
- C. Wang, C. Zhao, J. Sun, S. Huang, X. Liu and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2016–2023 CrossRef CAS PubMed.
- Y. L. Liu and C. I. Chou, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5267–5282 CrossRef CAS PubMed.
- I. Hamerton, L. T. Mcnamara, B. J. Howlin, P. A. Smith, P. Cross and S. Ward, Macromolecules, 2014, 47, 1946–1958 CrossRef CAS.
- A. D. Baranek, L. L. Kendrick, C. A. Tretbar and D. L. Patton, Polymer, 2013, 54, 5553–5559 CrossRef CAS PubMed.
- A. D. Baranek, L. L. Kendrick, C. A. J. Narayanan, G. E. Tyson, S. Wand and D. L. Patton, Polym. Chem., 2012, 3, 2892–2900 RSC.
- T. Agag, A. Akelah, A. Rehab and S. Mostafa, Polym. Int., 2012, 61, 124–128 CrossRef CAS PubMed.
- P. J. Flory, Polymer, 1970, 20, 1317–1320 CrossRef.
- H. Ishida and D. J. Allen, Polymer, 1996, 37, 4487–4495 CrossRef CAS.
- X. Li, Y. Xia, W. Xu, Q. Ran and Y. Gu, Polym. Chem., 2012, 3, 1629–1633 RSC.
- S. H. Hsiao and H. Y. Chang, J. Polym. Sci., Part A: Polym. Chem., 1996, 34, 1421–1431 CrossRef CAS.
- H. Ishida and D. J. Allen, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 1019–1030 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |