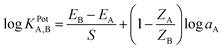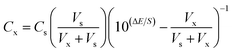Potentiometric multi-walled carbon nanotube Zn-sensor based on a naphthalocyanine neutral carrier: experimental and theoretical studies
Ola R. Shehab* and
Ahmed M. Mansour
Chemistry Department, Faculty of Science, Cairo University, Gamaa Street, Giza 12613, Egypt. E-mail: olashehab_chem@yahoo.com; Fax: +20 2 35728843; Tel: +20 2 01001905350
First published on 26th June 2015
Abstract
A new multi-walled carbon nanotube graphite paste sensor based on 2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine as a neutral carrier (2.0%), 2-flouorophenyl-2-nitrophenyl ether (50.0%) as a plasticizer, and sodium tetrakis-imidazolyl borate (1.0%) as an anionic additive has been explored as a selective sensor for determination of Zn2+ in real samples. The electrode showed a fast response time of 5 s, gave a Nernstian response (29.9 mV per decade) over the concentration range 1.0 × 10−8 to 1.5 × 10−4 mol L−1, and could be used in the pH range of 4.3–7.5 with a detection limit of 5.0 × 10−9 mol L−1. The response mechanism of the electrode was investigated using UV-vis and FT IR spectroscopy. Scanning electron microscopy combined with energy dispersive X-ray spectra were used to confirm the reaction between Zn2+ ions and naphthalocyanine on the surface of the electrode. In order to predict the selectivity of the naphthalocyanine sensor for different metal ions, the corresponding binding energies of the metal complexes were calculated at the Hartree–Fock level of theory.
1. Introduction
Chemically modified carbon paste electrodes (CMCPEs) have been successfully applied as potentiometric sensors for the determination of various species.1–4 Compared to the other types of ion selective electrodes (ISEs), CMCPEs are extremely simple to prepare, easy to regenerate, and give a stable response with a very low ohmic resistance,5,6 corresponding to the formation of a very thin film of a pasting liquid coated onto small particles of carbon powder.7 Multi-walled carbon nanotubes (MWCNTs) were discovered by Iijima,8 which have attracted great interest owing to their highly accessible surface area with a narrow size distribution, excellent electrical conductivity, and high stability. These properties encouraged analysts for using MWCNTs in the composition of sensors instead of normal graphite with a large particle size. The electrodes contained MWCNTs showed excellent features such as high sensitivity, low detection limit, and fast response that may be attributed to the signal enhancement provided by high surface area, low overvoltage, and rapid electrode kinetics.9 Moreover, an improvement in the electric conductivity and mechanical properties of the paste matrix was reported.In the present study, the choice of Zn2+ is ascribed for the presence of Zn2+ as a component of several enzymes such as carboanhydrases, and proteinases.10–12 It is the second most abundant transition metal ion in the human body and plays significant role in many biological processes such as brain function and pathology, immune function, gene transcription. In addition, Zn(II) has unique ability to promote rewinding of the melted DNA, and can stimulate hydrolysis of DNA and RNA.13 Zinc and its compounds are widely used in electroplating, paint, rubber, dye, wood preservatives, and batteries. However, its presence in high protein foods and its large doses can cause fever, chills, pulmonary manifestation, gastroenteritis, vomiting, nausea, anemia and renal failure. Besides, a major problem is the subsequent pollution and harm to the environment and humans because of its frequent use.14 For example, common zinc compounds such as zinc chloride, zinc oxide, zinc sulfate and zinc sulfide are found at hazardous waste sites. In view of its toxicity, the determination of Zn2+ becomes more important. Several analytical techniques have been used for determination of Zn2+ such as flame atomic absorption spectrometry,15 fluorimetry,16 UV-vis,17 ICP-AES,18 potentiometry,19–25 and voltammetry.26
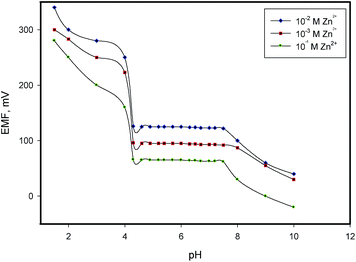
Macrocycles are most likely to be used in ISEs, however not all macrocycles are useful as sensing electrodes. They should provide high complexation or extraction selectivity for a particular metal ion and enough conformational flexibility for fast ion exchange. Besides that they must have high lipophilicity to remain in membrane and moderate molecular weight to allow high mobility. The function and applications of metallonaphthalocyanine coordination compounds are exciting as it expands from them being catalytic centers, through photosensitizing agents in therapy and other applications, to key cofactors in several biological systems. The selected macrocyclic ligand in this study (naphthalocyanine motif) has attracting interest in the field of construction and design of new sensor capable of detecting definite metal ion of compatible dimensions in his electron rich heart cavity.27,28 Based on various parameters, the high stability and selectivity of its metal ion complex, its solubility and high ability to extract the metal ion into the paste phase, 2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine was explored as an active material in carbon paste electrode for the fabrication of Zn2+ selective sensor. Comparison between the proposed electrode and the reported Zn2+ sensors based on macrocyclic ionophores27–35 showed that the developed sensor has superiority over the reported electrodes in terms of low detection limit and fast response time (Table 1).
| Ion-recognition | Slope, mV | Linear range, M | LOD, M | Response time, s | Ref. |
|---|---|---|---|---|---|
| 5,10,15,20-Tertraphenyl-21H,23H-porphine | 29.0 | 6.2 × 10−6 to 1.0 × 10−1 | — | 12 | 31 |
| Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid | 30.0 | 1.3 × 10−5 to 1.0 × 10−1 | — | 10 | 30 |
| Hematoporphyrin IX | 28.6 | 5.0 × 10−5 to 1.0 × 10−1 | — | 30 | 33 |
| Protoporphyrin IX | 29.0 | 1.5 × 10−5 to 1.0 × 10−1 | — | 32 | |
| Benzo-substituted macrocyclic diamide | 30.0 | 9.0 × 10−5 to 1.0 × 10−1 | 5.0 × 10−5 | 20 | 34 |
| Bzo2Me2Ph2(16)hexaeneN4 | 28.5 | 2.8 × 10−6 to 1.0 × 10−1 | 2.2 × 10−6 | <10 | 35 |
| 12-Crown-4 | 29.5 | 7.0 × 10−5 to 1.0 × 10−1 | — | 10 | 27 |
| Triaza-5,8-dioxo-3(4),9(10)-dibenzoyl-1,12,14-triene | 29.2 | 1.3 × 10−7 to 1.0 × 10−1 | 1.0 × 10−8 | 7 | 29 |
| Thiazolidin-4-one | 29.0 | 9.2 × 10−5 to 1.0 × 10−2 | 2.0 × 10−7 | 12 | 28 |
| Tetra-tert-butyl-2,3-naphthalocyanine | 29.9 | 1.0 × 10−8 to 1.5 × 10−4 | 5.0 × 10−9 | 5 | This work |
2. Experimental
2.1. Chemicals
Graphite powder, 2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine, sodium tetraphenylborate (NaTPB), sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaTFPB), sodium tetrakis imidazolyl borate (NaTImB), dibutyl-butyl phosphonate (DBBP), o-nitrophenyloctyl ether (o-NPOE), dioctyl adipate (DOA), tricresyl phosphate (TCP), 2-flouorophenyl-2-nitrophenyl ether (FPNPE), and THF were purchased from Aldrich chemical company. MWCNT (3–20 nm OD, 1–3 nm ID, 0.1–10 micro long) was purchased from Alfa Aesar. NaNO3, KNO3, NH4NO3, Cd(NO3)2, Ba(NO3)2, Ni(NO3)2·6H2O, Pb(NO3)2, Mg(NO3)2·6H2O, Mn(NO3)2·4H2O, Al(NO3)3·9H2O, Cu(NO3)2·3H2O, and Ca(NO3)2·4H2O were purchased from Oxford, India.2.2. Apparatus
Digital Jenway 3010, and 3505 pH meters were used for potential, pH measurements, respectively. A SENTEK R1/2MM Ag/AgCl electrode was used as the outer reference electrode. UV-vis spectra were recorded on a Shimdazu Lambda 4B spectrophotometer. FT IR-460 plus JASCO 4000–400 cm−1 was used for IR measurements (KBr pellets & solutions in THF). Perkin-Elmer Optima 2000 ICP instrument was used for the atomic emission spectrometry measurements. Quanta FEG 250 instrument was used to obtain the scanning electron micrographs (SEM) of the electrodes.2.3. Sensors preparation
The modified paste was prepared by mixing the appropriate weight of naphthalocyanine, and highly pure graphite with acetone. The mixture is homogenized by careful mixing with agate pestle in agate mortar, left at room temperature to evaporate acetone, and then a weighed amount of the plasticizer is added. Similar, the MWCNT sensor was prepared by the same way with the addition of the MWCNT to the graphite powder. The paste is then packed into the electrode body.36 All EMF measurements were carried out with the following cell assembly:Ag/AgCl, KCl (3 M)/test solution/filling graphite modified paste/carbon paste electrode.
2.4. Selectivity of the sensor
Potentiometric selectivity factor (KPotA,B) was evaluated using the separate solution method (SSM)37 according to the following relation:where EA, EB are the electrode potentials of 1.0 × 10−3 mol L−1 solution of each of Zn2+ and interfering cation, ZA, ZB are the charges on Zn2+ and the interfering cations, respectively and S is the theoretical slope.
2.5. Potentiometric determination
Standard addition method was applied for the potentiometric determination.38 In this method, known amounts of standard Zn2+ were added to a sample of 25.0 mL with the unknown concentration and from the potential change, ΔE = (Eu − Es) one can determine the concentration of the test sample using the equation:where Cx is the unknown concentration, Vx is the volume of sample solution, Vs and Cs are the volume and concentration of the standard Zn2+ solution added to the sample, respectively, ΔE is the change in potential after addition of certain volume of standard solution, and S is the slope of the calibration graph.
2.6. Computational details
Full-unconstrained geometry optimization of 2,11,20,29-tetra-tert-butyl-2,3-naphthalocyanine and its complexes have been carried out at Hartree–Fock (HF) level of theory. In the model structures of naphthalocyanine, the naphthalene and tertiary butyl groups were replaced by H atoms36 to reduce the computer time. The standard 6-31G(d) basis set was used for Na+, Mg2+, Al3+, K+, Ca2+, Mn2+, Ni2+, Cu2+, and Zn2+, while the effective-core potential (ECP) of LANL2DZ was applied for Cd2+, Ba2+ and Pb2+. The effect of solvent on the theoretical parameters was performed using the default Polarized Continuum Model (PCM),39 where water as a solvent was considered as a uniform medium (ε = 78.39). Ionization energy, electron affinity, hardness, softness, dipole moment, and energy gap were calculated according to Koopman's theorem.40 Natural bonding orbitals (NBO) analysis41 of Mn2+, Ni2+, Cu2+, and Zn2+ complexes were performed using NBO 3.1 program at the HF/6-31G(d) level of theory. All the calculations were carried out by Gaussian 03 package.423. Results and discussion
3.1. Factors affecting the performance of the Zn2+ sensor
| No. | %composition | Slope (mV) | linear range (M) | Detection limit (M) | Relative standard deviationb% | |||
|---|---|---|---|---|---|---|---|---|
| Ionophore | Graphite | Plasticizer | Additive | |||||
| a M: multiwalled carbon nanotube.b Average of four replicates. | ||||||||
| 1 | 2 | 49.0 | 49.0NPOE | — | 43.0 | 2.5 × 10−4 to 5.0 × 10−3 | 1.60 × 10−4 | 1.12 |
| 2 | 3 | 49.0 | 48.0NPOE | — | 55.0 | 3.2 × 10−4 to 3.2 × 10−3 | 1.00 × 10−4 | 1.21 |
| 3 | 1 | 49.5 | 49.0NPOE | 0.5NaTFPB | 37.1 | 7.9 × 10−6 to 3.2 × 10−3 | 7.90 × 10−6 | 1.45 |
| 4 | 2 | 48.5 | 49.0NPOE | 0.5NaTFPB | 33.2 | 3.9 × 10−6 to 5.0 × 10−3 | 3.80 × 10−6 | 0.24 |
| 5 | 3 | 48.5 | 47.5NPOE | 0.75NaTFPB | 50.5 | 1.9 × 10−5 to 2.5 × 10−3 | 9.50 × 10−6 | 0.34 |
| 6 | 2 | 48.5 | 49.0NPOE | 0.5NaTImB | 29.9 | 3.9 × 10−6 to 3.9 × 10−3 | 2.13 × 10−6 | 0.33 |
| 7 | 2 | 48.5 | 49.0NPOE | 0.5NaTPB | 16.7 | 1.3 × 10−6 to 5.0 × 10−3 | 1.09 × 10−6 | 2.13 |
| 8 | 2 | 48.5 | 49.0DBP | 0.5NaTImB | 17.6 | 7.9 × 10−6 to 5.0 × 10−3 | 5.88 × 10−6 | 2.56 |
| 9 | 2 | 48.5 | 49.0DOA | 0.5NaTImB | 39.7 | 5.0 × 10−5 to 1.0 × 10−2 | 4.16 × 10−5 | 2.44 |
| 10 | 2 | 48.5 | 49.0TCP | 0.5NaTImB | 24.7 | 1.3 × 10−6 to 5.0 × 10−4 | 1.25 × 10−6 | 1.22 |
| 11 | 2 | 48.5 | 49.0DBBP | 0.5NaTImB | 55.7 | 1.6 × 10−4 to 5.0 × 10−3 | 7.94 × 10−5 | 1.78 |
| 12 | 2 | 48.5 | 49.0FPNPE | 0.5NaTImB | 26.6 | 3.9 × 10−7 to 5.0 × 10−3 | 3.98 × 10−7 | 0.66 |
| 13 | 2 | 48.0 | 49.0FPNPE | 1.0NaTImB | 29.3 | 2.0 × 10−7 to 1.5 × 10−3 | 2.00 × 10−7 | 0.44 |
| 14 | 2 | 47.0 | 50.0FPNPE | 1.0NaTImB | 28.7 | 3.9 × 10−7 to 9.3 × 10−4 | 3.90 × 10−7 | 0.33 |
| 15 | 2 | 46.5 + 0.5 M | 50.0FPNPE | 1.0NaTImB | 29.5 | 5.0 × 10−8 to 1.3 × 10−5 | 5.01 × 10−8 | 0.21 |
| 16 | 2 | 46.0 + 1.0 M | 50.0FPNPE | 1.0NaTImB | 29.9 | 1.0 × 10−8 to 1.5 × 10−4 | 5.01 × 10−9 | 0.23 |
| 17 | 2 | 45.5 + 1.5 M | 50.0FPNPE | 1.0NaTImB | 29.5 | 5.0 × 10−8 to 1.5 × 10−4 | 1.00 × 10−8 | 0.36 |
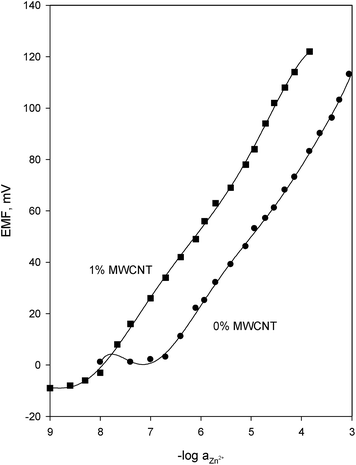
Since the use of plasticizers gives some permeable properties to the paste and may improve the mechanical stability of the sensor,47 so it was necessary to study the response characteristics of the electrode in terms of the nature of plasticizer.43,48,49 For this, different types of plasticizers, NPOE (ε = 23.6), DBP (ε = 6.4), DOA (ε = 3.9), TCP (ε = 6.9), DBBP (ε = 4.6), and FPNPE (ε = 50.0) were examined. As shown in Table 2, the best performance was observed for the electrodes plasticized with NPOE and FPNPE with slopes 29.9, and 29.3 mV, in that order. The highest dielectric constant of FPNPE may be the main reason for decreasing the detection limit of the investigated electrode to 2.0 × 10−7 mol L−1. Therefore, FPNPE was chosen as the best plasticizer for the working electrode. Alternatively, highly accessible surface area with a narrow distribution size, excellent electrical conductivity, and high stability of MWCNT attract the authors to explore the performance of the naphthalocyanine electrode in terms of different amount of the added MWCNT. As shown in Table 2 (no. 16) and represented in Fig. 1, the electrode doped with 1% MWCNT showed great enhanced sensitivity observed by the lower detection limit, 5.0 × 10−9 mol L−1 compared with its analogue without MWCNT (no. 14).
| Temperature (°C) | Slope (mV per decade) | Linear range (M) | ||
|---|---|---|---|---|
| 0% MWCNT | 1% MWCNT | 0% MWCNT | 1% MWCNT | |
| 25 | 29.96 | 29.50 | 3.98 × 10−7 to 1.28 × 10−2 | 1.00 × 10−8 to 1.47 × 10−4 |
| 30 | 30.95 | 29.98 | 4.57 × 10−7 to 5.49 × 10−2 | 1.00 × 10−8 to 1.50 × 10−4 |
| 40 | 32.79 | 34.76 | 3.98 × 10−7 to 1.14 × 10−2 | 1.00 × 10−8 to 1.00 × 10−4 |
| 50 | 33.10 | 35.85 | 3.98 × 10−7 to 3.54 × 10−2 | 1.00 × 10−8 to 1.11 × 10−4 |
| Cation | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KPotZn,J KPotZn,J |
|
|---|---|---|
| With 1% MWCNT | Without MWCNT | |
| Zn2+ | 0.00 | 0.00 |
| Na+ | −7.12 | −5.00 |
| NH4+ | −6.51 | −3.72 |
| K+ | −7.37 | −4.51 |
| Cd2+ | −2.23 | −1.55 |
| Ba2+ | −1.35 | −1.62 |
| Ca2+ | −1.45 | −1.71 |
| Ni2+ | −1.25 | −1.75 |
| Pb2+ | −3.51 | −0.34 |
| Mg2+ | −1.18 | −1.44 |
| Mn2+ | −3.15 | −7.65 |
| Cu2+ | −2.03 | 0.37 |
| Al3+ | −1.35 | 2.70 |
| Fe2+ | −1.32 | −0.03 |
| Fe3+ | −0.76 | 0.37 |
| Metal ion | Binding energy |
|---|---|
| Na+ | −146.657 |
| Mg2+ | −75.6891 |
| Al3+ | −291.089 |
| K+ | −392.712 |
| Ca2+ | −294.171 |
| Mn2+ | −65.1576 |
| Ni2+ | −19.3597 |
| Cu2+ | −44.4163 |
| Zn2+ | −77.4744 |
| Cd2+ | −454.393 |
| Ba2+ | −1039.63 |
| Pb2+ | −478.316 |
3.2. Response mechanism
The mechanism of the investigated Zn2+ sensor was explored by observing the electronic absorption spectra of the naphthalocyanine ionophore in THF before and after the reaction with 0.1 M Zn2+ solution. Porphyrins are characterized by intense and sharp absorption bands in the range of 400–500 nm referred as Soret or B-band. In the range of 500–700 nm, the electronic spectra of porphyrins and their naphthalocyanine derivatives show a group of bands named Q-bands.52 The electronic absorption spectrum of the titled ionophore displayed several bands in the UV-region at 210, 245, 280, 325, and 360 nm as well as a shoulder at 405 nm. Besides, a strong band at 780 nm and two shoulders at 695 and 735 nm are observed in the visible range (Fig. 5). By adding Zn2+ ions to the naphthalocyanine solution, a clean renovation to new species was observed as evidenced by a blue shifted of the Q-band to 760 nm and the disappearance of the band at 360 nm. Another attempt to investigate the mechanism of the electrode towards Zn2+ was obtained using solution FT IR analysis (in THF). The IR spectrum of the free ionophore (Fig. 6(A)) showed a medium band at 1628 cm−1 assigned to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). This mode was shifted to lower wave number and overlapped with the C
N). This mode was shifted to lower wave number and overlapped with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrational modes in the same region with the appearance of the band at 1563 cm−1. Therefore, the C
C vibrational modes in the same region with the appearance of the band at 1563 cm−1. Therefore, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups are involved in chelation (Fig. 6(B)). Assignment of the NH group before and after adding Zn2+ ions to naphthalocyanine solution is not easy owing to presence of H-bonds between ionophore and THF molecules. For this, the spectrum of the paste containing the ionophore, graphite, FPNPE, and NaTImB was compared to the spectrum of the same paste after keeping in contact with 0.1 M Zn2+ for 1 h and then rinsed with water for 10 s. Comparison between the solid spectra indicated that NH groups are involved in complexation formation as the ν(NH) mode is shifted from 3425 to 3416 cm−1 upon contact with Zn2+. Therefore, naphthalocyanine interacted with Zn2+ as a neutral carrier through four coordination centers.
N groups are involved in chelation (Fig. 6(B)). Assignment of the NH group before and after adding Zn2+ ions to naphthalocyanine solution is not easy owing to presence of H-bonds between ionophore and THF molecules. For this, the spectrum of the paste containing the ionophore, graphite, FPNPE, and NaTImB was compared to the spectrum of the same paste after keeping in contact with 0.1 M Zn2+ for 1 h and then rinsed with water for 10 s. Comparison between the solid spectra indicated that NH groups are involved in complexation formation as the ν(NH) mode is shifted from 3425 to 3416 cm−1 upon contact with Zn2+. Therefore, naphthalocyanine interacted with Zn2+ as a neutral carrier through four coordination centers.
Scanning electron microscope (SEM) is a valuable tool used for characterizing the surface morphology of the sensor. As shown in Fig. 7, the appearance of the metallic zinc on the surface of the sensor having the titled ionophore after its dipping in a solution of Zn2+ for one hour could be taken as evidence for the surface reaction. Energy dispersive X-ray (EDX) is a key tool used for identification of sample, since each element has a unique atomic structure that allow unique set of peaks on its X-ray spectrum. The growing of a sharp and intense peak for zinc at about 8.6 keV in the EDX spectrum of the surface of the electrode after dipping in zinc solution (Fig. 7) is a sign for the successful reaction of the titled ionophore with the Zn2+ ions at the electrode surface.
3.3. Quantum chemical calculations
The most essential property for metal ion selectively with naphthalocyanine is the binding energy (B.E.). Binding energies are defined as the total energy of complex minus the sum of total energies of the most stable isolated moieties, i.e., metal ion, and free naphthalocyanine. The energies of the free metal ions and naphthalocyanine have been calculated in the aqueous phase at Hartree–Fock (HF) level of theory. The standard 6-31G(d) basis set was used for Na+, Mg2+, Al3+, K+, Ca2+, Mn2+, Ni2+, Cu2+, and Zn2+, while LANL2DZ was applied for Cd2+, Ba2+ and Pb2+. The calculated values of binding energies are given in Table 5. The binding energies sequences follow the order Ba2+ > Pb2+ > Cd2+ > K+ > Ca2+ > Al3+ > Na+ > Zn2+ > Mg2+ > Mn2+ > Cu2+ > Ni2+. The titled ionophore has a large flexible cavity, which can accommodate a variety of metal ions to form stable coordination compounds. The interference coming from the stable isoelectronic configurations of neon (Na+ and Al3+), argon (K+ and Ca2+), and xenon (Ba2+), Cd2+ ([Kr]4d10), as well as Pb2+ ([Xe]4f145d106s26p2) might be attributed to several explanations. First, it is necessary to clarify that our calculations were performed on a single molecule in the aqueous phase, rather than the experimental measurements which were carried out on the bulk solution. Second, the solvent properties such as hydrogen-bond ability and solute–solvent interactions cannot be provided by PCM model and these variables are well known to affect the selectivity of the titled ionophore, and also the value of formation constant. The stereochemistry of the formed metal complexes may be one of the reasons which did not be considered in the calculations as our assumption was based on the formation of square-planar geometry. In quantum chemical calculations, it is difficult to take into consideration several variables such as the complexity of the paste (additives, plasticizer, etc.) and electrode–solution interface. Finally, the energies values of the free interfering cations were found to be lower than Zn2+ ion, while the corresponding complexes have higher energies than the zinc ionophore complex, despite these ions have little ability towards the formation of metal complexes owing to the absence of incomplete d-orbitals.Natural bond orbital (NBO) analysis was carried out for the complexes of Mn, Ni, Cu, and Zn to shed more light about the preference of the investigated naphthalocyanine towards Zn among the transition metal ions. NBO can provide details about the type of hybridization, the nature of bonding and strength of the interactions between metal ion and donor sites.41 According to NBO, the electronic configurations of Mn, Ni, and Cu in naphthalocyanine complexes are [Ar]4s0.133d5.204p0.19, [Ar]4s0.253d8.154p0.25, and [Ar]4s0.293d9.754p0.21 comparing with [Ar]4s0.253d9.954p0.27 for the Zn complex. The calculated natural charges were found to be 1.478e, 1.346e, 0.752e, and 1.528e for Mn, Ni, Cu, and Zn in that order. The quantity of electron density donation from the donor sites of the ionophore to the metal ions is not the same. The natural charges are more reduced in case of Ni and Cu comparing with Mn and Zn as the half- and completely filled d-orbitals, which give extra stability to these complexes. The increase in the binding energy of Zn2+ with respect to the other transition metal elements may be assigned to the back donation from the completely filled d-orbitals to the empty p-orbitals in the titled ionophore.
The energies and compositions of the frontier molecular orbitals53 are important properties in several chemical and pharmacological processes. EHOMO is associated with the electron donating ability, while ELUMO indicates the ability of the molecule to accept electrons. The chemical hardness and softness of a molecule as good indicator for the chemical reactivity of a given molecule can be calculated from the energy values of EHOMO and ELUMO. HOMO–LUMO gap (ΔE) decides whether the molecule is hard or soft; a soft molecule is more polarizable than the hard one. Hard molecules need small energy to excitation. Softness (S) is a property of molecule that measures the extent of chemical reactivity. It was found that Mn, Ni, Cu, and Cd complexes are harder than Zn(II) complex. Dipole moment (μ in Debye) was used to qualitatively analyze the trend in the hydrophobic values (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P). A very significant dipole moment may polarize the molecule in such a way that it produces a required potential at several atomic centers necessary for binding and activity. Herein, the higher dipole moment values were reported for Pb, Al, Ca, and Zn in that order. Fig. 8 shows the optimized structures of free ionophore, and zinc-ionophore complex; HOMO and LUMO of zinc-ionophore complex.
P). A very significant dipole moment may polarize the molecule in such a way that it produces a required potential at several atomic centers necessary for binding and activity. Herein, the higher dipole moment values were reported for Pb, Al, Ca, and Zn in that order. Fig. 8 shows the optimized structures of free ionophore, and zinc-ionophore complex; HOMO and LUMO of zinc-ionophore complex.
 | ||
| Fig. 8 Optimized structures of (a) free ionophore, and (b) zinc-ionophore complex; (c) HOMO of zinc-ionophore complex, and (d) LUMO of zinc-ionophore complex. | ||
3.4. Analytical applications
| Taken (μg mL−1) | Found (μg mL−1) | Recovery% | Relative standard deviationa% | |
|---|---|---|---|---|
| Pure samples | 0.49 | 0.50 | 98.00 | 1.02 |
| 0.98 | 1.00 | 98.00 | 1.12 | |
| 2.99 | 3.00 | 99.60 | 1.04 | |
| 4.99 | 5.00 | 99.80 | 1.10 |
| 2K4[Fe(CN)6] + 3Zn(NO3)2 = K2Zn3[Fe(CN)6]2 + 6KNO3 |
3.5. Validation of the proposed electrode
The reproducibility and stability of the electrode were investigated by repeating the preparation and the calibration of each composition four times. As shown in Table 1, the RSD% values were found to be in the range of 0.21–2.56%. The potential readings for the electrode no. 16 dipped in 1.0 × 10−4 mol L−1 Zn2+ solution ten times are negligibly changed (±0.5 mV) over 4 h, which indicates the good reproducibility and high stability of the electrode. The accuracy was expressed in terms of percentage deviation of the measured concentration from the actual concentration. The obtained results (Table 6) are within the acceptance range of 3%.The ruggedness of the potentiometric method was carried out by constructing the calibration curve using the same working solution, electrode, and experimental conditions over four days, and with four different persons. The RSD% values were found to be less than 1% for the repetitive experiments in four days, and <2% for the repetitive experiments by the different workers. The obtained data revealed that the method is reproducible.
4. Conclusion
2,11,20,29-Tetra-tert-butyl-2,3-naphthalocyanine as a neutral carrier contained in a modified carbon paste electrode has been used for the determination of Zn2+ ions (in the nano-molar levels) in the real samples such as multivitamin drug, Prisoline zinc eye drop and water samples (tap water and granite factory). Addition of a multi-walled carbon nanotube (MWCNT) to this electrode resulted in a great enhanced sensitivity observed by the lower detection limit, 5.0 × 10−9 mol L−1. Besides, this sensor has higher electrical conductivity and higher resistance to interferents. High sensitivity, and selectivity, a wide range, a low detection limit, and very fast response time are the significant properties of the investigated electrodes. The increase in the binding energy of Zn2+ with respect to the other transition metal elements may be assigned to the back donation from the completely filled d-orbitals to the empty p-orbitals in the titled ionophore.References
- A. Abbaspour and S. M. M. Moosavi, Chemically modified carbon paste electrode for determination of copper(II) by potentiometric method, Talanta, 2002, 56, 91–96 CrossRef CAS.
- H. M. Abu-Shawish and S. M. Saadeh, A New Chemically Modified Carbon Paste Electrode for Determination of Copper Based on N,N′ Disalicylidenehexameythyl-enediaminate Copper(II) Complex, Sens. Lett., 2007, 5, 565–571 CrossRef CAS PubMed.
- K. Kalcher, J. M. Kauffmann, J. Wang, I. Svancara, K. Vytras, C. Neuhold and Z. Yang, Sensors Based on Carbon Paste in Electrochemical Analysis: A Review with Particular Emphasis on the Period 1990–1993, Electroanalysis, 1995, 7, 5–22 CrossRef CAS PubMed.
- J. Pei, Q. Yin and J. Zhong, Potentiometric determination of trace silver based on the use of a carbon paste electrode, Talanta, 1991, 38, 1185–1189 CrossRef CAS.
- I. Svancara and K. Schachi, Testing of umodified carbon paste electrodes, Chem. Listy, 1999, 93, 490–499 CAS.
- K. Vytras, J. Kalous and J. Jezkova, Automated potentiometry as an ecologic alternative to twophase titrations of surfactants, Egypt. J. Anal. Chem., 1997, 6, 107–123 CAS.
- I. Svancara, M. K. Hvizdalova, K. Vytras, K. Kalcher and R. Novotny, A microscopic study on carbon paste electrodes, Electroanalysis, 1996, 8, 61–65 CrossRef CAS PubMed.
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 1991, 354, 56–58 CrossRef CAS PubMed.
- M. M. Ardakani and M. A. Sheikh-Mohseni, Carbon Nanotubes - Growth and Applications, ed. M. Naraghi, InTech, 2011 Search PubMed.
- Y. H. Chiu, G. J. Gabriel and J. W. Canary, Ternary ligand-zinc-hydroxamate complexes, Inorg. Chem., 2005, 44, 40–44 CrossRef CAS PubMed.
- T. A. Miller, D. J. Witter and S. J. Belvedere, Histone deacetylase inhibitors, J. Med. Chem., 2003, 46, 5097–5116 CrossRef CAS PubMed.
- A. Scozzafava and C. J. Supuran, Carbonic anhydrase and matrix metalloproteinase inhibitors sulphonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulphonamide inhibit both these zinc enzymes, J. Med. Chem., 2000, 43, 3677–3687 CrossRef CAS PubMed.
- S. K. Miller, D. G. van-Derveer and L. G. Marzilli, Models for the interaction of Zn-2+ with DNA – the synthesis and X-ray structural characterization of 2 octahedral Zn complexes with monomethyl phosphate-esters of 6-oxopurine 5′-monophosphate nucleotides, J. Am. Chem. Soc., 1985, 107, 1048–1055 CrossRef CAS.
- A. L. Nolan, M. J. McLaughlin and S. D. Mason, Chemical speciation of Zn, Cd, Cu, and Pb in pore waters of agricultural and contaminated soils using donnan dialysis, Environ. Sci. Technol., 2003, 37, 90–98 CrossRef CAS.
- Q. Li, X. H. Zhao, Q. Z. Lv and G. G. Liu, The determination of zinc in water by flame atomic absorption spectrometry after its separation and preconcentration by malachite green loaded microcrystalline triphenylmethane, Sep. Purif. Technol., 2007, 55, 76–81 CrossRef CAS PubMed.
- M. Hosseini, Z. Vaezi, M. R. Ganjali, F. Faridbod, S. D. Abkenar, K. Alizadeh and M. Salavati-Niasari, Fluorescence “turn-on” chemosensor for the selective detection of zinc ion based on Schiff-base derivative, Spectrochim. Acta, Part A, 2010, 75, 978–982 CrossRef PubMed.
- P. Kaur, S. Kaur, A. Mahajan and K. Singh, Highly selective colorimetric sensor for Zn2+ based on hetarylazo derivative, Inorg. Chem. Commun., 2008, 11, 626–629 CrossRef CAS PubMed.
- P. Wilhartitz, S. Dreer, R. Krismer and O. Bobleter, High performance ultra-trace analysis in molybdenum and tungsten accomplished by on-line coupling of ion chromatography with simultaneous ICP-AES, Mikrochim. Acta, 1997, 125, 45–52 CrossRef CAS.
- A. R. Fakhari, M. Shamsipur and K. H. Ghanbari, Zn(II)-selective membrane electrode based on tetra(2-aminophenyl) porphyrin, Anal. Chim. Acta, 2002, 460, 177–183 CrossRef CAS.
- M. B. Gholivand and Y. Mozaffari, PVC-based bis(2-nitrophenyl)disulfide sensor for zinc ions, Talanta, 2003, 59, 399–407 CrossRef CAS.
- N. R. Gupta, S. Mittal, S. Kumar and S. K. A. Kumar, Potentiometric studies of N,N′-Bis(2-dimethylaminoethyl)-N,N′-dimethyl-9,10 anthracenedimethanamine as a chemical sensing material for Zn(II) ions, Mater. Sci. Eng., C, 2008, 28, 1025–1030 CrossRef CAS PubMed.
- V. K. Gupta, S. Agarwal, A. Jakob and H. Lang, A zinc-selective electrode based on N,N′-bis(acetylacetone)ethylenediimine, Sens. Actuators, B, 2006, 114, 812–818 CrossRef CAS PubMed.
- V. K. Gupta, A. K. Jain and G. Maheswari, A new Zn2+-selective potentiometric sensor based on dithizone—PVC membrane, Chem. Anal., 2006, 51, 889–897 CAS.
- M. Hosseini, S. D. Abkenar, M. R. Ganjali and F. Faridbod, Determination of zinc(II) ions in waste water samples by a novel zinc sensor based on a new synthesized Schiff's base, Mater. Sci. Eng., C, 2011, 31, 428–433 CrossRef CAS PubMed.
- P. Singh, A. K. Singh and A. K. Jain, Electrochemical sensors for the determination of Zn2+ ions based on pendant armed macrocyclic ligand, Electrochim. Acta, 2011, 56, 5386–5395 CrossRef CAS PubMed.
- X. Lu, Z. Wang, Z. Geng, J. Kang and J. Gao, 2,4,6-tri(3,5-Dimethylpyrazoyl)-1,3,5-triazine modified carbon paste electrode for trace Cobalt(II) determination by differential pulse anodic stripping voltammetry, Talanta, 2000, 52, 411–416 CrossRef CAS.
- V. K. Gupta, A PVC-based 12-crown-4 membrane potentiometric sensor for zinc(II) ions, Sens. Actuators, B, 1999, 55, 195–200 CrossRef CAS.
- V. K. Gupta, M. Al Khayat, A. K. Minocha and P. Kumar, Zinc (II) - selective sensors based on dibenzo-24-crown-8 in PVC matrix, Anal. Chim. Acta, 2005, 532, 153–158 CrossRef CAS PubMed.
- S. Chandra and D. R. Singh, Zinc(II) selective poly(vinyl chloride) membrane ISE using a macrocyclic compound 1,12,14-triaza-5,8-dioxo-3(4),9(10)-dibenzoylcyclopentadeca-1,12,14-triene as neutral carrier, J. Saudi Chem. Soc., 2010, 14, 55–60 CrossRef CAS PubMed.
- V. K. Gupta, D. K. Chauhan, V. K. Saini, S. Agarwal, M. M. Antonijevic and H. Lang, A porphyrin based potentiometric sensor for Zn2+ determination, Sensors, 2003, 3, 223–235 CrossRef CAS PubMed.
- V. K. Gupta, A. K. Jain, R. Mangla and P. Kumar, A new Zn2+-selective sensor based on 5,10,15,20-tetraphenyl-21H,23H-porphine in PVC Matrix, Electroanalysis, 2001, 13(12), 1036–1040 CrossRef CAS.
- V. K. Gupta, A. Kumar and R. Mangla, Protoporphyrin IX dimethyl ester as active material in PVC matrix membranes for the fabrication of zinc(II) selective sensor, Sens. Actuators, B, 2001, 76, 617–623 CrossRef CAS.
- A. K. Jain, S. M. Sondhi and S. Rajvanshi, A PVC based hematoporphyrin IX membrane potentiometric sensor for zinc(II), Electroanalysis, 2002, 14, 293–296 CrossRef CAS.
- M. Shamsipur, S. Rouhani, M. R. Ganjali, H. Sharghi and H. Eshghi, Zinc-selective membrane potentiometric sensor based on a recently synthesized benzo-substituted macrocyclic diamide, Sens. Actuators, B, 1999, 59, 30–34 CrossRef CAS.
- A. K. Singh, A. K. Jain, P. Saxena and S. Mehtab, Zn(II)-selective membrane electrode based on tetraazamacrocycle [Bzo2Me2Ph2(16)hexaeneN4], Electroanalysis, 2006, 18, 1186–1192 CrossRef CAS PubMed.
- O. R. Shehab and A. M. Mansour, New thiocyanate potentiometric sensors based on sulfadimidine metal complexes: Experimental and theoretical studies, Biosens. Bioelectron., 2014, 57, 77–84 CrossRef CAS PubMed.
- Y. Umezawa, P. Buhlmann, K. Umezawa, K. Tohda and S. Amemiya, Potentiometric selectivity coefficients of ion-selective electrodes Part I. Inorganic cations - (Technical report), Pure Appl. Chem., 2000, 72, 1851–2082 CrossRef CAS.
- E. Lindner and Y. Umezawa, Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report), Pure Appl. Chem., 2008, 80, 85–104 CrossRef CAS.
- N. T. Abdel Ghani and A. M. Mansour, Molecular structures of 2-arylaminomethyl-1H-benzimidazole: Spectral, electrochemical, DFT and biological studies, Spectrochim. Acta, Part A, 2012, 91, 272–284 CrossRef CAS PubMed.
- T. A. Koopmans, Ordering of wave functions and energies to the individual electrons of an atom, Physica, 1933, 1, 104–113 CrossRef CAS.
- A. M. Mansour, Crystal structure, DFT, spectroscopic and biological activity evaluation of analgin complexes with Co(II), Ni(II) and Cu(II), Dalton Trans., 2014, 15950–15957 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, GAUSSIAN 03 (Revision A.9), Gaussian, Inc., Pittsburgh, 2003 Search PubMed.
- E. Bakker, P. Buhlmann and E. Pretsch, Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics, Chem. Rev., 1997, 97, 3083–3132 CrossRef CAS PubMed.
- V. A. Nicely and J. I. Dye, A General Purpose Curvefitting Program for Class and Research Use, J. Chem. Educ., 1971, 48, 443–448 CrossRef.
- E. Ammann, P. Pretsch, W. Simon, E. Lindner, A. Bezegh and E. Pungor, Lipophilic salts as membrane additives and their influence on the properties of macro- and micro-electrodes based on neutral carriers, Anal. Chim. Acta, 1985, 171, 119–129 CrossRef.
- R. Eugster, P. M. Morf, U. Spichiger and W. Simon, Selectivity-modifying influence of anionic sites in neutral-carrier-based membrane electrodes, Anal. Chem., 1991, 63, 2285–2289 CrossRef CAS.
- E. A. Cummings, P. Mailley, S. Linquette-Mailley, B. R. Eggins, E. T. McAdams and S. McFadden, Amperometric carbon paste biosensor based on plant tissue for the determination of total flavanol content in beers, Analyst, 1998, 123(10), 1975–1980 RSC.
- R. Eugster, U. E. Spichiger and W. Simon, Membrane model for neutral-carrier-based membrane electrodes containing ionic sites, Anal. Chem., 1993, 65, 689–695 CrossRef CAS.
- X. Yang, N. Kumar, H. Chi, H. Chi, D. B. Hibbert and P. N. W. Alexandr, Lead-selective membrane electrodes based on dithiophenediazacrownether derivatives, Electroanalysis, 1997, 9, 549–553 CrossRef CAS PubMed.
- L. I. Antropov, Theoritical Electrochemistry, Mir Publisher, Moscow, 1972 Search PubMed.
- T. Rostzin, E. Bakker, K. Suzuki and W. Simon, Lipophilic and immobilized anionic additives in solvent polymeric membranes of cation-selective chemical sensors, Anal. Chim. Acta, 1993, 280, 197–208 CrossRef.
- S. Ishihara, K. Minami, J. Labuta, J. P. Hill, W. V. Rossom, K. Ariga and D. Ishikawa, Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes, Phys. Chem. Chem. Phys., 2014, 16, 9713–9746 RSC.
- I. Fleming, Frontier Orbitals and Organic Chemical Reactions, Wiley, London, 1976 Search PubMed.
- S. Sadeghi, M. Eslahi, M. A. Naseri, H. Naeimi, H. Sharghi and A. Shameli, Copper Ion Selective Membrane Electrodes Based on Some Schiff Base Derivatives, Electroanalysis, 2003, 15, 1327–1333 CrossRef CAS PubMed.
- M. Qureshi, J. P. Rawat and T. Kaur, Determination of microgram quantities of zinc by oscillometric titration with potassium ferrocyanide, J. Electroanal. Chem., 1970, 26, 409–411 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |