Evaluation of the interactions between rosmarinic acid and bovine milk casein
Vincenza Ferraro†
a,
Ana Raquel Madureira *a,
Pedro Fontec,
Bruno Sarmentobc,
Ana M. Gomesa and
Manuela E. Pintadoa
*a,
Pedro Fontec,
Bruno Sarmentobc,
Ana M. Gomesa and
Manuela E. Pintadoa
aCBQF – Centro de Biotecnologia e Química Fina/Escola Superior de Biotecnologia, Universidade Católica Portuguesa, Rua Arquitecto Lobão Vidal, Apartado 2511, 4202-401 Porto, Portugal. E-mail: rmadureira@porto.ucp.pt; Tel: +351-225580044
bI3S, Instituto de Investigação e Inovação em Saúde, Universidade do Porto, Portugal and INEB – Instituto de Engenharia Biomédica, Universidade do Porto, Rua do Campo Alegre, Portugal
cCESPU, Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde, Rua Central de Gandra 1317, 4585-116 Gandra-PRD, Portugal
First published on 7th October 2015
Abstract
Polyphenols can interact with proteins, which gives rise to a significant loss of their biological properties. The objective of this research was the study of interactions in model systems composed of the polyphenol rosmarinic acid (RA) and bovine milk α-s1-casein, β-casein and κ-casein. Radical cation quenching assay (ABTS, 2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonic-acid), optical density, liquid chromatography (RP-HPLC, reverse phase-high performance liquid chromatography, and SEC, size exclusion chromatography), dynamic light scattering (DLS) and zeta-potential, Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC) were used for the screening of the interactions at 0, 3 and 24 h of storage time and at the refrigeration temperature 4 °C. Interactions were assessed at the pH of the complexes in water, 6.8, and at acidic pH 3 and 4.5. Results showed the occurrence of non-covalent interactions such as hydrophobic, hydrogen bonding and dipole–dipole. Radical cation quenching activity of RA significantly decreased in the presence of caseins, meaning that the amount of free RA diminished. Higher and the same degree of interaction were observed for α-s1-casein and β-casein. Complex dimensions were different depending on pH, time and on the primary and secondary structure of caseins. Interactions were shown to be favoured at the lowest pH, where complexes are biggest, and reversible at all pH conditions tested. The results of this study must be complemented with the analysis of more complex systems to take into account the effect of other milk components – lipids, sugars and minerals – on the interaction of RA.
1. Introduction
Protein, polyphenols and polysaccharides are the main constituents of plants, where they are present in a very specific conformation and location inside the intracellular environment. Usually, these compounds do not interact with one another since their compartments are separated.1 However, when disruption of tissues occurs, such as during plant processing for food and drink application, several phenomena can take place. The intracellular compounds confined in the cytoplasm and organelles come into contact with one another and with the extracellular molecules, and thus the polyphenols can bind to proteins and polysaccharides, and especially to those of the cell wall. Adsorption, solubilisation, migration, and oxidation of protein, polyphenols and polysaccharides (due to the contact with the ambient atmosphere), have as a consequence the rearrangement in new structures through different kind of interactions.2 In recent years and after the first works published by Haslam and collaborators,3,4 interactions between polyphenols and proteins, have gained increased attention due to their importance in the stability and properties of many food systems. Indeed, interaction between proteins and polyphenols has a significant impact on the nutritional and organoleptic characteristic of food.1,2Rosmarinic acid is a tannin, actually an ester of caffeic acid with 3,4-dihydroxyphenyl lactic acid, and most notably found in the family of Lamiaceae. Tannins are phenolic compounds able to precipitate alkaloids and globular proteins.5 Plant phenols can interact covalently or non-covalently with proteins; both ways can lead to the precipitation of proteins, via either multisite interactions (several phenolics bound to one protein molecule) or multidentate interactions (one phenolic bond to several protein sites or protein molecules).6 Non-covalent interactions between phenols and proteins likely arise from hydrophobic and van der Waals forces, and may be subsequently stabilized by hydrogen bonding. These interactions are reversible and do not alter the secondary structure of a protein (however they modify the solution properties of the protein). On the contrary, covalent interactions permanently modify the secondary structure of a protein. Reversible associations may result or not in protein precipitation depending on factors such as ionic strength, pH and solvent. Also, protein precipitation that occurs at low ratios protein to polyphenolic may be reversed as this ratio increases.7
Protein–phenol interactions are likely to modify bioavailability of both proteins and phenols.1 Actually, incorporation of polyphenols in dairy food is being considered as a way to develop oral nutraceutical formulation due to the antioxidant and anti-inflammatory activity. Alongside this, milk is one of the most complete sources of nutrients and is widely recognized as a beneficial food for a healthy growth of children and adults. However, as the bioavailability and the nutraceutical effects of many phenolic compounds are modified in the presence of proteins,2 it is of fundamental importance to understand the nature of the protein–phenolic interaction in dairy products to obtain the maximum benefit of these phenolic compounds.
Therefore, the objective of this research was the evaluation of the nature and extent of interactions occurring in model systems composed of the polyphenol rosmarinic acid and the bovine α-s1-casein, β-casein and k-casein. Some other papers have been published on this topic; however, they evaluated interaction at different concentrations and under different conditions. No papers have been reported on the interaction of RA with bovine milk caseins considered individually.
2. Materials and method
2.1 Chemicals
Reagents used for the study of interactions were rosmarinic acid (abbreviated as RA) (360.31 Da, purity ≥ 96%) and the bovine milk α-s1-casein, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 Da, purity ≥ 70% (abbreviated as a-cas), β-casein, 23
380 Da, purity ≥ 70% (abbreviated as a-cas), β-casein, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983 Da, purity ≥ 98% (abbreviated as b-cas) and κ-casein, 19
983 Da, purity ≥ 98% (abbreviated as b-cas) and κ-casein, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 038 Da, purity ≥ 70% (abbreviated as k-cas). For the antioxidant activity (radical quenching) assay, reagents used were ABTS (2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonic-acid) and potassium persulfate. For HPLC-PDA (high performance liquid chromatography with photo-diode-array detection) analysis, HPLC grade methanol and formic acid were used. For the SEC-UV/Vis (size exclusion chromatography with ultraviolet and visible detection) KH2PO4, K2HPO4 and NaCl were used for the mobile phase. Reagents for buffers used to study the effect of pH on interactions were acetic acid, sodium acetate, sodium citrate dehydrate and citric acid. All compounds and solvents were purchased from Sigma-Aldrich (Sintra; Portugal).
038 Da, purity ≥ 70% (abbreviated as k-cas). For the antioxidant activity (radical quenching) assay, reagents used were ABTS (2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonic-acid) and potassium persulfate. For HPLC-PDA (high performance liquid chromatography with photo-diode-array detection) analysis, HPLC grade methanol and formic acid were used. For the SEC-UV/Vis (size exclusion chromatography with ultraviolet and visible detection) KH2PO4, K2HPO4 and NaCl were used for the mobile phase. Reagents for buffers used to study the effect of pH on interactions were acetic acid, sodium acetate, sodium citrate dehydrate and citric acid. All compounds and solvents were purchased from Sigma-Aldrich (Sintra; Portugal).
2.2 Model solutions of caseins and rosmarinic acid
Model solutions were prepared taking into account the normal concentration of caseins in milk and the concentration of RA in the sage (Salvia officinalis) aqueous extract (infusion). Therefore, the RA–a-cas solution was prepared by adding 15 mg mL−1 a-cas to 0.1 mg mL−1 RA; the RA–b-cas solution was prepared by adding 9 mg mL−1 b-cas to 0.1 mg mL−1 RA and the RA–k-cas solution was prepared by adding 3.5 mg mL−1 k-cas to 0.1 mg mL−1 RA, either in ultrapure water or buffers. Samples were prepared in duplicate and maintained at 4 °C, and protected from light during 24 h. The effect of time on possible interactions was assessed at three moments: at the instant time, and after 3 and 24 h of incubation. Buffers used were 0.2 M acetate, composed of acetic acid (6 g/500 mL) and sodium acetate (8.2 g/500 mL) for pH 4.5 (ionic strength I = 37 mM electron2), and 0.2 M citrate, composed of sodium citrate dehydrate (29.41 g/500 mL) and citric acid (19.21 g/500 mL) for pH 3 (ionic strength I = 1.95 mM electron2).2.3 Antioxidant activity
The ABTS assay was performed according to the method reported by Re et al.8 and with the aim to determine an eventually loss of antioxidant activity of RA due to a loss in the number of free RA molecules or active groups (–OH) by interaction with proteins. Mixtures assayed were the protein–phenol solutions at the time of preparation (0 h), and the protein–phenol solutions left to stand 3 h and 24 h, in the dark and at 4 °C. Antioxidant activity of RA and protein was also assessed at the same times and at same storing conditions. The peroxyl radical ABTS˙+ was generated by mixing 7 mM ABTS (2,2′-azinobis-3-ethyl-benzothiazoline-6-sulfonic-acid) (Sigma-Aldrich) in ultrapure water with 2.54 mM potassium persulfate (Sigma-Aldrich) in ultrapure water, in the proportion 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v); the mixture was then allowed to react during 16 h in the dark and at a room temperature. The working solution of ABTS˙+ was prepared daily by diluting the stock in ultrapure water so as the absorbance at 734 nm was in the range 0.700 ± 0.02 measured on a UV-1203 mini spectrophotometer (Shymazu, Frilabo; Porto, Portugal). A volume of 100 μL of sample was added to 1 mL of the working ABTS˙+ solution and absorbance was read at 734 nm at 0, 3, 6 and 12 h and then after 24 h for each sample. Results were expressed as percentage inhibition (I (%)) of ABTS˙+ as follows:
1 (v/v); the mixture was then allowed to react during 16 h in the dark and at a room temperature. The working solution of ABTS˙+ was prepared daily by diluting the stock in ultrapure water so as the absorbance at 734 nm was in the range 0.700 ± 0.02 measured on a UV-1203 mini spectrophotometer (Shymazu, Frilabo; Porto, Portugal). A volume of 100 μL of sample was added to 1 mL of the working ABTS˙+ solution and absorbance was read at 734 nm at 0, 3, 6 and 12 h and then after 24 h for each sample. Results were expressed as percentage inhibition (I (%)) of ABTS˙+ as follows:
 | (1) |
2.4 Optical density
Optical density was measured on a UV-1203 mini spectrophotometer (Shymadzu, Frilabo; Carnaxide, Portugal) at the wavelength 300, 400, 500 and 600 nm. Model solutions, as well as each protein and RA, were analysed at initial time (0 h).2.5 Complex dimension and zeta potential through dynamic light scattering (DLS)
Medium diameter of RA, proteins and RA–proteins complexes and the relative zeta potential were assessed using a ZetaSizer NanoZSP (Malvern, UK). Dynamic light scattering (DLS) was used to assess complexes diameter with the intensity distribution weighted according to the scattering intensity of each particle fraction or family. Data was validated only if the cumulants fit error was <0.005. Zeta potential was measured using Laser Doppler Anemometry (LDA). All analyses were carried out with an angle of 90° at 25 °C.2.6 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of RA, standard milk proteins and the complexes RA–a-cas, RA–b-cas and RA–k-cas, were collected in the mid infrared (MIR) region between 4000 and 600 cm−1; the equipment ABB MB3000, interfaced with the Horizon MB software (Paços de Arcos, Portugal) was used. The spectra were collected in the absorbance mode and for samples in the solid state, obtained by a lyophilisation of the model solutions. Complexes were analysed at 24 h. Each spectrum was the result of the average of 60 scans at 4 cm−1 resolution.2.7 Differential scanning calorimetry (DSC)
Differential scanning calorimetry was carried out with the equipment DSC-60 Shimadzu (Frilabo). Calibration of the equipment was done with reference materials zinc and indium. Before the analysis, all samples were lyophilized, and then an accurately weighted amount of 4–6 mg was placed into an aluminum pan and sealed. Complexes were analysed at 24 h. Therefore, each pan was heated up to 450 °C, under a flow of dry nitrogen gas at 40 mL min−1 and at a heating rate of 10 °C min−1.2.8 Chromatographic analysis
RP-HPLC for RA quantification was performed using a Beckman & Coulter 168 series system interfaced with a photo diode array detector (PDA 190–600 nm) (Beckman & Coulter; Fullerton, CA, USA). Samples were analysed at the time 0 h. All eluents were filtered through a 0.45 μm cellulose membrane (Millipore-Interface; Amadora, Portugal) and degassed in an ultrasound bath (Millipore-Interface) for 15 min prior to be used as mobile phases. Column used was the Waters Symmetry RP-HPLC C18 250 × 4.6 mm, 5 μm particles size and 100 Å mean pore dimension. Mobile phases was A 92.5% water, 5% methanol, 2.5% formic acid, and B 92.5% methanol, 5% water, 2.5% formic acid. Elution flow was 0.5 mL min−1 with the following gradient: constant 40% B for first 2 min, from 40 to 50% B in 10 min (from 2 to 12 min), and then 50% B constant for 5 min (from 12 to 17 min), increase from 50 to 80% B in 8 min (from 18 to 25 min) and then constant 80% up to the end of the run, 26 min. Detection wavelengths were 280 and 320 nm.SEC analysis was performed with the Pharmacia Biotech system, equipped with the LCC-501 Plus controller, P-500 model pumps, and UV-M II detector. Elution flow was 1 mL min−1 with a mobile phase of 0.1 M phosphate buffer at pH 7.4 and 200 mM NaCl, which was added to prevent non-specific binding of sample to the stationary phase. The column SUPEROSE 12 10 × 300 mm (1000–300.000 Da resolution) was used for the analysis, and detection wavelength was 280 nm. All determinations were carried out in triplicate.
2.9 Calculation and statistical analysis
Statistical significance of the difference between values was evaluated with the software STATISTICA v.9.0, by one-way analysis of variance (one-way ANOVA) at a confidence level of 95% (p < 0.05).3. Results and discussion
Results show evidence of interactions between RA and caseins, where the entity of complexation depended on protein itself and on pH. No precipitation was noticed after addition of protein to the RA solution at the pH 6.8 meaning the occurrence of soluble complexes. On the contrary, an increased turbidity was observed at the time of mixing by decreasing pH to 4.5 and 3. A small precipitate was present after 24 h for α- and β-casein, while the solution continued turbid. At lowest pH values there was stronger electrostatic attraction between the positively charged proteins (Table 1) and the negatively charged phenolic compound, which resulted in greater complexes. At same time the electrostatic repulsion between negatively charged molecules at the highest pH 6.8, resulted in the minimum interacted amount with RA. In spite to a stronger interaction between RA and caseins, turbidity is also to be attributed to protein aggregation due to the presence of salts in the buffers, and to the pH 4.5 of the buffer itself, which is near the isoelectric point.| αs1-casein | β-casein | κ-casein | |
|---|---|---|---|
| pI | 4.92–5.35 | 4.83–5.07 | 5.30–5.80 |
| pH | Net charge of the protein | ||
| 6.8 | (−) | (−) | (−) |
| 4.5 | (+) | (+) | (+) |
| 3 | (+) | (+) | (+) |
| Molar ratio RA/protein | 0.47 | 0.74 | 1.51 |
As reported by McManus, Davis, Lilley and Haslam3 and by von Staszewski, et al.,10 precipitation is depending not just on pH but also on the stoichiometry of the protein–polyphenol complex. The precipitation of protein by polyphenol, in fact, may be reversed by adding more protein in solution, and the complex, once formed, may also be dissociated by treatment with solvents such as acetone. When concentration of protein is lower than concentration of polyphenols, the polyphenol associates to one or more sites on the protein surface to give a mono-layer, which is less hydrophilic than the protein itself; aggregation and precipitation can then occur. On the contrary, when the protein concentration is higher, there is a tendency to cross-link proteins molecules by polyphenol bridges and precipitation can occur as well.11 Result of our study showed the occurrence of interactions by non-covalent bonds as discussed in the following paragraphs.
3.1 Antioxidant activity (radical scavenging)
The chemical antioxidant activity is important to determine since the interaction with proteins decreases the ability of phenolic compounds to quench free radicals.12,13 In fact, in the presence of polyphenols, the ABTS radical cation extracts from them an electron or hydrogen atom and quenches.14Antioxidant activity of complexes was significantly lower than the antioxidant activity of RA at the pH investigated (Fig. 1). At pH 6.8 and at the initial time, α-s1-casein and β-casein had the same and the highest degree of interaction with RA. By the loss of the antioxidant activity of RA it could be estimated that 58% of the phenol interacted with α-s1-casein and β-casein. Lower interaction was found in the case of κ-casein, 41%, where the result must be also attributed to the highest molar ratio RA to protein (Table 1). In fact, more phenol was available and then antioxidant activity was higher. As the time increased, and up to 24 h, the antioxidant activity of RA decreased significantly, due to oxidation to quinones, adducts formation, dimerisation and hydrolysis14 (Fig. 1). On the contrary, a slight decrease was observed in the case the complexes, and even an increase was noticed in the case of β-casein at pH 6.8. These results show that complexes are more stable than RA over the time considered. As expected, as the pH decreased the amount of RA which interacted with proteins increased due to stronger electrostatic attractions (Table 1), and particularly in the case of κ-casein. For instance, by lowering the pH from 6.8 to 3, the amount of RA interacted with protein increased from 58 to 66% in the case of α-s1-casein and β-casein, and from 41 to 55% in the case of κ-casein, at the initial time (Fig. 2). At acidic pH the antioxidant activity of polyphenols is higher; it decreases with increasing pH due to the deprotonation of the hydroxyl group. Also, polyphenols are mild acidic and resist on pH change because of their resonance; this is why they act as antioxidant in almost every conditions except very low or very high pH (<2 or >10) or temperatures.14
 | ||
| Fig. 1 Inhibition of ABTS˙+ by RA, by caseins and by complexes RA–caseins at pH 6.8, 4.5 and 3, at 4 °C and over 24 h. | ||
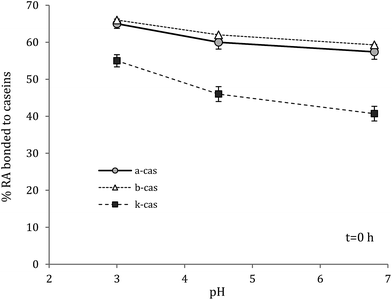 | ||
| Fig. 2 Effect of pH on the amount of RA bonded to caseins at t = 0 h and 4 °C, determined by the loss of antioxidant activity (i.e. by the ABTS assay). | ||
3.2 Optical density
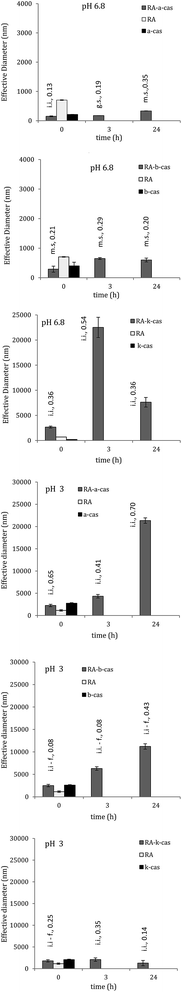
Result for optical density also showed occurrence of interaction between RA and caseins. Absorbance of complexes was in fact different than the sum of the absorbance of RA and protein, meaning that a new “species” took place as a consequence of interactions. In Fig. 3 is reported the optical density at pH 6.8, where the greatest value was observed for the complex RA–k-cas. That casein is strongly self-associating due to the presence of two cysteine residues, which on the contrary are absent in α-s1-casein and β-casein. Also, although it is globally hydrophilic (on the contrary of α-s1-casein and β-casein which are hydrophobic) it is the most hydrophobic in the aminic side, which gives rise to hydrophobic interactions.15 In the presence of RA, κ-casein molecules can further associate and be stabilised by hydrophobic and electrostatic interactions between the two compounds.16 The aggregation accounted for a greater particles diameter compared with α-s1- and β-casein complexes, as discussed in the following paragraph, and then in a higher optical density.3.3 Complex dimension and zeta potential analysis through DLS
Analysis of effective diameter of particles in solution (i.e. particles diameter + electrical double layer) and of zeta potential through the dynamic light scattering assay also revealed the existence of interactions. Effective diameter of complexes, dispersity and zeta potential were variable depending on casein composition and on pH (Fig. 4). As reported by some authors,17 polyphenols are not completely solubilised either in buffer or water; they form spherical aggregates of varying diameters, and same happens in the case of proteins. Caseins, in fact, form micelles and assemble either above or below the isoelectric point. The number of monomers per micelle can vary with the pH, being high at physiological conditions (20 monomers per micelle against 6 at acidic pH of 3).18 Those finding has been confirmed by our DLS measurements (Fig. 4) as discussed as follows. Regarding the polyphenol, it is calculated that each molecule of RA has a volume of 303.03 cubic Å, at which correspond a medium diameter of 8.34 Å, where the molecule not spherical.19 DLS measurements gave a medium diameter of 706 nm at pH 6.8, 957 nm at pH 4.5 and 1147 nm at pH 3; therefore, it could be estimated that aggregates are composed of 1700 RA molecules at pH 6.8, 2300 molecules at pH 4.5 and 2760 molecules at pH 3.In the case of complexes, those involving κ-casein had the greatest tendency to aggregate (as also showed by optical density measurements), followed by RA–b-cas. At pH 6.8, the effective diameter slightly increased over 24 h (p > 0.05) in the case of RA–a-cas, it was constant after 3 h and up to 24 h (p ≤ 0.05) in the case of RA–b-cas, while a very high increase was found for RA–k-cas after 3 h (p ≤ 0.05). Dimension of complexes significantly increased as the pH decreased. At pH 3, dimension of complexes significantly increased over 24 h (p ≤ 0.05) in the case of α-s1- and β-casein, while was constant in the case of κ-casein (p > 0.05) (Fig. 4).
Amino acids sequences determine the primary structure of a protein, while hydrophobic forces and hydrogen bond (and also sulphur bridges, when present) are responsible of the secondary, tertiary and quaternary structure. They define the degree of expansion or agglomeration, i.e. the higher those forces the higher the degree of protein agglomeration.10 This phenomenon was responsible for the dimension of the complexes, where protein aggregates trapped RA molecules inside a network created by hydrophobic forces and hydrogen bonds.16 As the pH of solution decreased from 6.8 to 3, dimension of the complex significantly increased due to the higher electrostatic attraction between RA and proteins, turning the complexes biggest, instable and near to flocculation (Fig. 4).
3.4 Fourier transform infrared spectroscopy (FTIR)
FTIR spectroscopy provides information about the secondary structure of protein. The technique works by shining infrared radiation on a sample, and seeing at which wavelengths the radiation is absorbed by covalent bonds. On the contrary, physical interactions are not detectable by FTIR.20 In our assays, in the case of complexes, no bands relative to the vibration of new covalent bonds with respect to casein where registered. FTIR spectra of complex were in fact identical to the FTIR spectra of protein, indicating that the polyphenol and the proteins interacted via non-covalent bonds (Fig. 5). There are three important regions in the analysis of FTIR spectra: the high frequency zone between 4000 and 1300 cm−1 which is the region of the functional groups, the region between 900 and 400 cm−1, and the region between 1300 and 900 cm−1 which is the finger-print zone, characteristic of the specific compound. The polypeptide and protein repeat units give rise to nine characteristic IR absorption bands, namely, amide A and B, and amide I to VII. Of these, the amide I and II are the two most prominent vibrational bands of the protein backbone.21 The amide I band between 1600 and 1700 cm−1 found in our study (Fig. 5) is mainly due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of peptide linkages (approximately 80% of the potential energy) and is directly related to the backbone conformation; amide I is also the most sensitive spectral region. Amide II, at approximately 1600–1450 cm−1, results from the N–H bending (40–60% of the potential energy) and from the C–N stretching (18–40%); this band is conformational sensitive. Amide III and IV are very complex bands resulting from a mixture of several coordinate displacements. It is possible to see an isolated group of characteristic peaks of proteins in the finger-print region (1300–900 cm−1) (Fig. 5); these peaks also appear in the case of complexes, at each pH investigated, meaning that no new covalent bonds between protein and RA were formed. It can be also noticed from Fig. 5 that absorbance of amides I and II was higher in the case of complexes, where the phenomena is attributed to the presence of more polar bonds in the complexes with respect to the caseins alone.21
O stretch of peptide linkages (approximately 80% of the potential energy) and is directly related to the backbone conformation; amide I is also the most sensitive spectral region. Amide II, at approximately 1600–1450 cm−1, results from the N–H bending (40–60% of the potential energy) and from the C–N stretching (18–40%); this band is conformational sensitive. Amide III and IV are very complex bands resulting from a mixture of several coordinate displacements. It is possible to see an isolated group of characteristic peaks of proteins in the finger-print region (1300–900 cm−1) (Fig. 5); these peaks also appear in the case of complexes, at each pH investigated, meaning that no new covalent bonds between protein and RA were formed. It can be also noticed from Fig. 5 that absorbance of amides I and II was higher in the case of complexes, where the phenomena is attributed to the presence of more polar bonds in the complexes with respect to the caseins alone.21
3.5 Differential scanning calorimetry (DSC)
DSC assays revealed the existence of non-covalent complexation between proteins and RA. In the case of α-s1-casein three globally exothermic events can be observed (Fig. 6, Table 2). The first globally exothermic event, at 60 °C, must be attributed to aggregation phenomena (exothermic process) which are a consequence of incipient thermally induced denaturation (endothermic process). Starting from that temperature caseins unfold and can interact one another by hydrophobic bonds.22 The second event registered at 88 °C is still relative to aggregation. The third globally exothermic peak, at 333 °C, is relative to melting (endothermic) and chemical decomposition through pyrolysis (exothermic). In the case of RA, it can be noticed an exothermic event at 169 °C relative to decomposition, i.e. oxidation, which gives rise to a number of breakdown products that further decompose;23 this originated several DSC peaks registered above that temperature (Fig. 6). The peak of RA oxidation at 169 °C disappeared in the case of the complex RA–a-cas; an exothermic event occurs at 128 °C after aggregation at 62 °C. This result should be attributed to the aggregation of unfolded protein with RA which probably contributed to stabilize the polyphenol; in fact, it is possible to notice that RA breakdown products disappeared in DSC of the complex (Fig. 6). | ||
| Fig. 6 DSC of RA (dot line), caseins (dash line) and complexes (solid line) at pH 4.5, 4 °C and 24 h. | ||
| DSC data | Peak | α-casein | RA–α-casein | RA |
|---|---|---|---|---|
| ΔH (cal g−1) | 1 | −15.4 | −27.3 | −29 |
| T (°C) | 60 | 62 | 169 | |
| ΔH (cal g−1) | 2 | −8.2 | −18.1 | |
| T (°C) | 88 | 128 | ||
| ΔH (cal g−1) | 3 | −31.3 | −27.5 | |
| T (°C) | 333 | 330 |
| β-casein | RA–β-casein | RA | ||
|---|---|---|---|---|
| ΔH (cal g−1) | 1 | −10.1 | −15 | −29 |
| T (°C) | 57 | 60 | 169 | |
| ΔH (cal g−1) | 2 | −5.2 | −9.1 | |
| T (°C) | 98 | 90 | ||
| ΔH (cal g−1) | 3 | −57.3 | −20.4 | |
| T (°C) | 332 | 329 | ||
| κ-casein | RA–κ-casein | RA | ||
|---|---|---|---|---|
| ΔH (cal g−1) | 1 | −15.1 | −22.3 | −29 |
| T (°C) | 57 | 60 | 169 | |
| ΔH (cal g−1) | 2 | −5.4 | −16.5 | |
| T (°C) | 112 | 138 | ||
| ΔH (cal g−1) | 3 | −31 | −14 | |
| T (°C) | 329 | 328 | ||
In the case of β-casein, an aggregation event was registered at 57 °C, being relative to hydrophobic bonds between unfolded protein molecules. The second event for β-casein was registered at 98 °C is still relative to aggregation. In the case of complex RA–b-cas it can be noticed a second exothermic event at 90 °C, which can be attributed to the interaction of denatured protein molecules with RA. The interaction contributed to stabilize the polyphenol by avoiding autoxidation as in the case of RA–a-cas. The third event at 333 °C for β-casein and at 329 °C for the complex is relative to melting (endothermic) and chemical decomposition through pyrolysis (exothermic). Same conclusion can be made in the case of κ-casein and relative complexes.
Analysing the enthalpy values (Table 2), it can be noticed that greater heats of aggregation are found in the case of complexes, meaning that aggregates of denatured casein with RA are more stable than aggregates of denatured caseins alone. Relatively to decomposition, complexes released less energy than the protein; the result arises from the lower heat capacity, due to a reduction in the number of bonds in complexes with respect to the caseins alone. Where fewer bonds are present there are also fewer degrees of freedom of motion, and then lower heat capacity. Therefore, less heat energy in the form of vibrations can be stored.24
Denaturation of milk caseins depends on pH, aside temperature, heating time and concentration. As reported by Akkerman,22 the rate of denaturation at acidic pH is significantly lower than at neutral pH, and protein–protein aggregates are made by non-covalent bonds such as hydrophobic interactions; this is why the disulphide bonds (present in κ-casein) are more stable at acidic conditions.23 In the light of these finding, complex at pH 6.8 showed a different behavior during heating, while the pH did not affected the stability of polyphenol (Fig. 7). Even if globally exothermic events were still noticed, peaks highness of complexes was smaller compared to pH 4.5. This means that denaturation occurred at greater extent, i.e. the endothermic heat of denaturation was higher compared to the exothermic heat of aggregation.
 | ||
| Fig. 7 DSC at pH 6.8 of RA–a-cas (solid line), RA–b-cas (dash line), RA–k-cas (dash-dot line) and RA (dot line). | ||
3.6 Chromatographic analysis
High performance liquid chromatography assays, performed on complexes at the time 0 h, also showed that interactions between RA and the milk caseins were determined by physical forces such as hydrophobic, dipole–dipole (also hydrogen bond) and van der Waals interactions. Solutions were filtered through a mesh of 0.45 μm, i.e. 450 nm. From Fig. 4 it is possible to see that, at time 0 h, effective diameter of complexes (which is not the “physical” diameter) is less than 450 nm for RA–a-cas and RA–b-cas, while is higher for RA–k-cas. At same time, particle size distribution is broader in the case of RA–k-cas, meaning that some complexes passed through the filter and then through the HPLC column. A proof of the presence of complexes in the filtrate has been the lower antioxidant activity, by ABTS, of 0.45 μm filtered RA–casein solutions with respect to a 0.45 μm filtered RA solution. HPLC assays used to determine the amount of free RA in solution showed that interactions with caseins were broken through the adsorption on the stationary phase, where the result is probably the sum of two phenomena. First, higher affinity of the phenol for the stationary phase than for proteins so as the amount of free RA detected corresponded to the whole RA amount before complexation; second, cleavage of protein by the formic acid present in the mobile phase,25 which can partially split the complex and release the polyphenol.Regarding SEC, the technique was used with the aim to estimate an increase in the native molecular weight of protein as effect of complexation with RA. However, chromatograms of complexes were same as standard proteins. The result must be partially attributed to the pH of the stationary phase, 7.4, at which α-s1-casein and β-casein and k-casein are negatively charged like RA, and then interactions were likely reversed. Also, proteins could interacted with the hydroxyl group of agarose, i.e. the stationary phase, and not just with the hydroxyl group of RA, as a result of ionic or ion-exchange interactions.26 It is worth to highlight, then, that HPLC and SEC are not suitable for the screening of complexation and must be therefore combined with other techniques for a more reliable result. In this regards, the micellar electrokinetic chromatography was proven to be useful to demonstrate the interaction of tea polyphenols with milk casein.27
4. Conclusions
Understanding of interactions of phenolic compounds with casein is an important subject as polyphenols may be potential food additives. Rosmarinic acid was able to interact with caseins through physical and reversible interactions. Complexes showed to be stable in solution, particularly at lower pH 3. At the concentration considered in this study, higher and same degree of interaction occurred between RA and α-s1-casein and RA and β-casein. Techniques used showed to be complementary one another; however, FTIR and HPLC can discriminate the nature of the interactions.Results of this study with model solutions will be complemented with further researches on more complex systems. These systems will take into account the presence of other essential elements present in the milk matrix. Lipids, sugar and metals, and also enzymes and biliary salts present in the gut, must be taken inconsideration in order to understand how they can influence the in vitro bioavailability of the phenol considered.
Acknowledgements
Authors acknowledge FCT – Fundação para a Ciência e Tecnologia for funding research work trough project NANODAIRY (PTDC/AGR-ALI/117808/2010) and by National Funds from FCT through project PEst-OE/EQB/LA0016/2013. Author Ana Raquel Madureira acknowledges FCT for the post-doctoral scholarship SFRH/BPD/71391/2010.References
- C. le Bourvellec and C. Renard, Interactions between polyphenols and macromolecules: quantification methods and mechanisms, Crit. Rev. Food Sci. Nutr., 2012, 52(3), 213–248 CrossRef CAS PubMed.
- Z. Hassan, H. M. F. El Din, A. Ali, N. S. Mehanna and T. El-Messery, Interaction of some low molecular weight phenolics with milk proteins, World Appl. Sci. J., 2013, 23(2), 182–187 CAS.
- J. P. McManus, K. G. Davis, T. H. Lilley and E. Haslam, The association of proteins with polyphenols, J. Chem. Soc., Chem. Commun., 1981, 7, 309b–311 RSC.
- E. Haslam, T. H. Lilley and L. G. Butler, Natural astringency in foodstuffs—a molecular interpretation, Crit. Rev. Food Sci. Nutr., 1988, 27(1), 1–40 CrossRef CAS PubMed.
- S. V. Prigent, H. Gruppen, A. J. Visser, G. A. van Koningsveld, G. A. de Jong and A. G. Voragen, Effects of non-covalent interactions with 5-O-caffeoylquinic acid (chlorogenic acid) on the heat denaturation and solubility of globular proteins, J. Agric. Food Chem., 2003, 51(17), 5088–5095 CrossRef CAS PubMed.
- H. M. Rawel, K. Meidtner and J. Kroll, Binding of selected phenolic compounds to proteins, J. Agric. Food Chem., 2005, 53(10), 4228–4235 CrossRef CAS PubMed.
- G. Luck, H. Liao, N. J. Murray, H. R. Grimmer, E. E. Warminski, M. P. Williamson, T. H. Lilley and E. Haslam, Polyphenols, astringency and proline-rich proteins, Phytochemistry, 1994, 37(2), 357–371 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radicals Biol. Med., 1999, 26, 1231–1237 CrossRef CAS.
- H. Farrell, R. Jimenez-Flores, G. Bleck, E. Brown, J. Butler, L. Creamer, C. Hicks, C. Hollar, K. Ng-Kwai-Hang and H. Swaisgood, Nomenclature of the proteins of cows' milk—sixth revision, J. Dairy Sci., 2004, 87(6), 1641–1674 CrossRef CAS.
- M. von Staszewski, F. L. Jara, A. L. Ruiz, R. J. Jagus, J. E. Carvalho and A. M. Pilosof, Nanocomplex formation between β-lactoglobulin or caseinomacropeptide and green tea polyphenols: impact on protein gelation and polyphenols antiproliferative activity, J. Funct. Foods, 2012, 4(4), 800–809 CrossRef CAS PubMed.
- K. J. Siebert, N. V. Troukhanova and P. Y. Lynn, Nature of polyphenols–proteins interactions, J. Agric. Food Chem., 1996, 44, 80–85 CrossRef CAS.
- K. M. Riedl and A. E. Hagerman, Tannins-proteins complexes as radical scavengers and radical skins, J. Agric. Food Chem., 2001, 49, 4917–4923 CrossRef CAS PubMed.
- H. Rawel, J. Kroll and U. Hohl, Model studies on reactions of plant phenols with whey proteins, Food Nahrung, 2001, 45(2), 72–81 CrossRef CAS.
- R. B. Walker and J. D. Everette, Comparative reaction rates of various antioxidants with ABTS radical cation, J. Agric. Food Chem., 2009, 57(4), 1156–1161 CrossRef CAS PubMed.
- L. K. Creamer, J. E. Plowman, M. J. Liddell, M. H. Smith and J. P. Hill, Micelle stability: κ-casein structure and function, J. Dairy Sci., 1998, 81(11), 3004–3012 CrossRef CAS.
- E. Jöbstl, J. R. Howse, J. P. A. Fairclough and M. P. Williamson, Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy, J. Agric. Food Chem., 2006, 54(12), 4077–4081 CrossRef PubMed.
- L. Pohjala and P. Tammela, Aggregating behavior of phenolic compounds—a source of false bioassay results?, Molecules, 2012, 17(9), 10774–10790 CrossRef CAS PubMed.
- I. Portnaya, E. Ben-Shoshan, U. Cogan, R. Khalfin, D. Fass, O. Ramon and D. Danino, Self-assembly of bovine β-casein below the isoelectric pH, J. Agric. Food Chem., 2008, 56(6), 2192–2198 CrossRef CAS PubMed.
- ChemAxon, Rosmarinic acid: Properties Viewer, ChemAzon, Cambridge M.A., 2015 Search PubMed.
- M. I. Kaldas, U. K. Walle, H. van der Woude, J. M. McMillan and T. Walle, Covalent binding of the flavonoid quercetin to human serum albumin, J. Agric. Food Chem., 2005, 53(10), 4194–4197 CrossRef CAS PubMed.
- J. Kong and S. Yu, Fourier transform infrared spectroscopic analysis of protein secondary structures, Acta Biochim. Biophys. Sin., 2007, 39(8), 549–559 CrossRef CAS PubMed.
- M. Akkerman. The effects of heating processes on milk whey protein denaturation and rennet coagulation processes. Master thesis, Aarhus University, DK, 2012.
- A. A. Hamama and W. W. Nawar, Thermal decomposition of some phenolic antioxidants, J. Agric. Food Chem., 1991, 39(6), 1063–1069 CrossRef CAS.
- J. Smith, Introduction to chemical engineering thermodynamics, J. Chem. Educ., 1950, 27(10), 584 CrossRef.
- B. Meyer, D. G. Papasotiriou and M. Karas, 100% protein sequence coverage: a modern form of surrealism in proteomics, Amino Acids, 2011, 41(2), 291–310 CrossRef CAS PubMed.
- D. Sheehan, Physical biochemistry: principles and applications. John Wiley & Sons, 2009 Search PubMed.
- L. Kartsova and A. Alekseeva, Effect of milk caseins on the concentration of polyphenolic compounds in tea, J. Anal. Chem., 2008, 63(11), 1107–1111 CrossRef CAS.
Footnote |
| † Present address: INRA – Institut National de la Recherche Agronomique, UR 1268 BIA – Biopolymères, Interactions, Assemblages, 44000 Nantes, UR 370 QuaPA – Qualité de Produits Animaux, 63000 Clermont-Fd, France. |
| This journal is © The Royal Society of Chemistry 2015 |