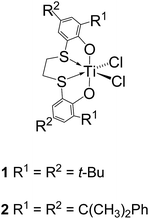
Styrene–isoprene and styrene–1,3-pentadiene copolymerisation catalyzed by titanium [OSSO]-type catalysts†
Marianna Loria,
Antonio Proto and
Carmine Capacchione*
Dipartimento di Chimica e Biologia and NANOMATES Research Centre for NANOMAterials and nanoTEchnology, Università degli Studi di Salerno, via Giovanni Paolo II 132, 84084 Fisciano, SA, Italy. E-mail: ccapacchione@unisa.it
First published on 28th July 2015
Abstract
The synthesis of styrene–isoprene and styrene–1,3-pentadiene binary copolymers promoted by the titanium complexes dichloro{1,4-dithiabutanediyl-2,2′-bis(4,6-di-tert-butylphenoxy)}titanium (1) and dichloro{1,4-dithiabutanediyl-2,2′-bis[4,6-bis(2-phenyl-2-propyl)phenoxy]}titanium (2) activated by methylaluminoxane (MAO) is reported. Both copolymers were obtained in a wide range of compositions and the molecular weight distributions obtained from GPC analysis of the copolymers are coherent with the material being copolymeric in nature. DSC analysis shows an increase of Tg by increasing the amount of styrene in the copolymers, for both binary copolymers. Furthermore, 13C NMR analysis of the copolymer microstructure allowed the assessment of the monomer block lengths and distribution in the polymer chain revealing a random distribution with the catalyst 1 and 2 for both binary copolymers and a low tendency to form long styrene homosequences. Intriguingly, both in styrene–isoprene and in styrene–1,3-pentadiene copolymers, the catalyst 2 produces polymers with higher vinyl (3,4 for isoprene and 1,2 for 1,3-pentadiene) content with respect to catalyst 1 giving interesting insights about the mechanism of stereocontrol for this class of catalysts.
Introduction
Copolymerisation of monomers with chemically distinct characteristics is a powerful tool to obtain new polymeric materials possessing unique chemical and physical properties with respect to the corresponding homopolymers. Styrene–butadiene (SB) copolymers represent, in this area, by far the most relevant industrial commodity. In particular, styrene–butadiene rubber (SBR) obtained by free radical emulsion processes and the thermoplastic block copolymers (SBS) obtained by solution processes based on living anionic polymerisation catalyzed by alkyllithium compounds are of fundamental importance in tyre production.1 Nevertheless, these commercial SB copolymers possess a low degree of stereoregularity both in styrene sequences and in diene segments because the anionic and radical polymerisation techniques do not allow precise control over the regiochemistry and stereochemistry during the growth of the polymeric chain.2In this context, the implementation of a polymerisation process based on transition metal complexes, that promote the polymerisation via coordination-insertion mechanism, is highly desirable because the possibility to control, in this case, both the regiochemistry and stereochemistry during the polymerisation.3
Indeed, the most covered route to synthesize stereoregular SB copolymers is the use of a titanium catalyst active in the syndiospecific polymerisation of styrene.4 In particular, the highly active catalysts based on half-titanocene have been widely used in the copolymerisation of styrene with butadiene. However, the direct polymerisation of styrene with conjugated dienes in the presence of group 4 complexes often face difficulties due to the completely different behaviour of the two classes of monomers for a given catalytic system that leads to a lowering of the catalyst activity and selectivity.
More recently the attention has shifted to rare-earth based catalyst that allows the synthesis of various diblock and multiblock copolymers based on styrene and conjugated dienes such as butadiene and isoprene.5
In our hands the [OSSO]-type titanium complex dichloro{1,4-dithiabutanediyl-2,2′-bis(4,6-di-tert-butylphenoxy)}titanium 1 has shown to be versatile catalyst for the copolymerisation of 1,3-alkadienes with styrene and ethylene giving, in some cases, copolymers with unprecedented microstructural features.6
In particular we have shown that the catalyst 1 activated by methylaluminoxane is able to efficiently copolymerize butadiene with styrene6d and p-methylstyrene with isoprene and butadiene.6i Furthermore the catalyst 1 also promote the polymerisation of the E isomer of 1,3-pentadiene giving a polymer consisting mainly of trans-1,2 units with a minor amount of 1,4 units.6h
Besides, the related more steric demanding complex dichloro{1,4-dithiabutanediyl-2,2′-bis[4,6-bis(2-phenyl-2-propyl)phenoxy]}titanium 2 bearing two cumyl groups, when activated by methylaluminoxane promote the living, isospecific polymerisation of styrene and permits the synthesis of SB di-block copolymers by sequential monomer addition.7
Here we report on the ability of the complexes 1 and 2 when activated by methylaluminoxane to efficiently copolymerise styrene with isoprene (I) and 1,3-pentadiene (PD) giving the corresponding copolymers in a wide range of composition and the complete characterization of the resulting products (Scheme 1).
Experimental part
Materials
All air sensitive compounds were manipulated under a nitrogen atmosphere using standard Schlenk techniques or a MBraun drybox. Toluene (Sigma-Aldrich) was dried over calcium chloride and refluxed 48 h under nitrogen atmosphere over sodium before using. Styrene was stirred over calcium hydride for one night and then purified by distillation under reduced pressure. Isoprene and E/Z-1,3-pentadiene (Sigma-Aldrich) were purified by distillation after stirring over calcium hydride for one night. Methylaluminoxane (MAO) was purchased from Sigma-Aldrich as a 10 wt% solution in toluene and used without further purification for the polymerisations involving isoprene and 1,3-pentadiene. The titanium complexes 1 and 2 were prepared according to the literature procedure.8Copolymerisation of styrene and isoprene
The copolymerisation of isoprene and styrene was carried out as follows. A 100 mL glass flask equipped with magnetic stirrer was charged with toluene (40 mL), isoprene (10 mL), a proper amount of styrene and MAO (15 mL; 0.023 mol). After equilibration of the solution at the polymerisation temperature (50 °C), a toluene solution (2 mL) of the catalyst (22 μmol) was injected. After 1 h, the reaction was stopped with ethanol (15 mL) containing hydroquinone as antioxidant. The polymer was coagulated in ethanol (200 mL) acidified with aqueous HCl, washed with an excess of ethanol, recovered by filtration and dried in vacuo at 40 °C.Copolymerisation of styrene and 1,3-pentadiene
The copolymerisation of 1,3-pentadiene and styrene was carried out as follows. A 100 mL glass flask equipped with magnetic stirrer was charged with toluene (15 mL for 1, 40 mL for 2), 1,3-pentadiene (5 mL), a proper amount of styrene and MAO (1.31 g; 0.023 mol). After equilibration of the solution at the polymerisation temperature (50 °C), a toluene solution (2 mL) of the catalyst (19 μmol of 1, 22 μmol of 2) was injected. After 1 h (for catalyst 2) or 3 h (for catalyst 1), the reaction was stopped with ethanol (15 mL) containing hydroquinone as antioxidant. The polymer was coagulated in ethanol (200 mL) acidified with aqueous HCl, washed with an excess of ethanol, recovered by filtration and dried in vacuo at 40 °C.Characterization of the polymers
The 13C NMR spectra of the polymer samples were recorded with a Bruker AVANCE 400 MHz spectrometer, using 5 mm o.d. NMR tubes in which about 30 mg of polymer samples were dissolved in CDCl3 (0.7 mL) and analyzed at room temperature. Chemical shifts were referred to TMS. The microstructure of the copolymer samples was assessed by comparing the observed resonances with the data from literature. The thermal analysis of the polymers were carried out on TA Instruments DSC Q20 using a heating rate of 10 °C min−1.The average molecular weights of the polymer samples were determined at 35 °C by a 150 Waters GPC equipped with JASCO 875-UV (254 nm) detector and four PSS columns set consisting of 106, 105, 104 and 103 Å (pore size) with 10 μm (particle size) column. THF was used as carrier solvent with a flow rate of 1.0 mL min−1. The calibration curve was established with polystyrene standards.
Results and discussion
Copolymerisation of styrene with isoprene
The peculiar behaviour of such catalytic systems in the copolymerisation of dienes and styrene and the scarcity, in the scientific literature, of catalytic systems able to promote the binary copolymerisation of styrene monomers with dienes other than 1,3-butadiene stimulated us to investigate the behaviour of the catalytic systems 1/MAO and 2/MAO in the copolymerisation of styrene with isoprene and 1,3-pentadiene.In Table 1 the results relative to the styrene–isoprene copolymers in the presence of both catalysts are presented. Firstly, the catalysts 1 and 2 were employed in the isoprene homopolymerisation (runs 1 and 7). Notably while the catalyst 1 produces polyisoprene with prevalently trans-1,4 (91%) and a lower amount of 3,4-units (9%) with a negligible content of cis-1,4 units, unexpectedly the more steric demanding catalyst 2 shows a lower degree of stereoselectivity with a higher degree of 3,4 (29%) and cis-1,4-insertion (18%) along the polymer chain (run 7). This different behaviour can be ascribed to the increased steric bulk around the metal centre of the complex 2 respect to the complex 1 and the concomitant presence of the methyl group in the 2-position of the isoprene molecule that enforce a 3,4 coordination of the monomer following the classical Cossee mechanism as for the α-olefins, instead of the allylic mechanism, resulting in the formation of the vinylic (3,4) units.10
| Entrya | Catalyst | [S]/[I] | Yield g (%) | Compositionc (mol%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | I1,4 (trans/cis) | I3,4 | Mw (× 103 Da) | Mw/Mn | Tg (°C) | ns | ||||
| a Polymerisation conditions: 22 μmol of complex, 0.1 mol of isoprene, 14.6 mL of MAO (Al/Ti = 1000), 40 mL of toluene, polymerisation time: 1 h, temperature: 50 °C.b 0.02 mol of isoprene.c Determined by 1H and 13C NMR analysis. | ||||||||||
| 1 | 1 | — | 0.03 (3) | — | 91 (91/0) | 9 | 25 | 1.4 | −62.9 | — |
| 2 | 1 | 0.02 | 0.1 (2) | 33 | 60 (56/4) | 7 | 21 | 1.5 | −6.2 | 1.7 |
| 3 | 1 | 0.03 | 0.2 (2) | 40 | 53 (48/5) | 7 | 23 | 1.5 | 5.9 | 2.0 |
| 4 | 1 | 0.05 | 0.6 (8) | 77 | 22 (21/1) | 1 | 32 | 1.3 | 42.7 | 2.6 |
| 5 | 1 | 0.1 | 1.6 (21) | 86 | 14 (13/1) | — | 46 | 1.5 | 54.2 | 6.5 |
| 6b | 1 | 1.0 | 1.7 (49) | 98 | 2 (2/0) | — | 43 | 1.7 | 80.4 | 35 |
| 7 | 2 | — | 0.02 (1) | — | 71 (53/18) | 29 | 75 | 1.1 | −64.9 | — |
| 8 | 2 | 0.02 | 0.1 (1) | 27 | 45 (19/26) | 28 | 6.0 | 1.2 | 25.7 | 1.3 |
| 9 | 2 | 0.03 | 0.1 (1) | 36 | 39 (27/12) | 25 | 7.0 | 1.2 | n.d. | 1.5 |
| 10 | 2 | 0.05 | 0.1 (2) | 66 | 13 (9/4) | 21 | 14 | 1.5 | 39.4 | 2.1 |
| 11 | 2 | 0.1 | 0.3 (4) | 75 | 9 (7/2) | 16 | 28 | 1.4 | 52.0 | 3.0 |
| 12 | 2 | 0.2 | 1.1 (12) | 92 | 6 (4/2) | 2 | 84 | 1.3 | 71.7 | 6.3 |
| 13 | 2 | 1.0 | 10.5 (61) | 97 | 3 (3/0) | — | 284 | 1.6 | 88.5 | 34 |
The copolymerisation of styrene with isoprene was performed using the 1 and 2 activated by MAO obtaining copolymers in a wide range of compositions (xs = 0.27–0.98; Table 1). Gel permeation chromatography (GPC) analysis of these copolymers showed that the molecular weights distributions are monomodal with a PDI values ranging from 1.3 to 1.7 consistent with their copolymeric nature. The molecular weight, in both cases, increases by increasing the styrene content and for the copolymers with styrene content higher than 86% is sensibly higher for the polymers obtained with the catalyst 2 (run 12 and 13) vis-à-vis catalyst 1 (run 5 and 6).
All copolymers are completely soluble in hexane, THF, or chloroform, also when the styrene concentration approaches as high values as xs = 0.98. As already observed for the p-methylstyrene–isoprene copolymers, the Tg values increase by increasing the styrene content ranging from −6.2 °C in the case of the copolymer containing the higher isoprene content (run 2), to a value close to the polystyrene Tg for the polymers containing higher content of styrene (runs 6 and 13). The stereoselectivity properties of the catalyst 1 and 2 displayed in the homopolymerisation of styrene and isoprene are retained in the copolymerisation reactions. Indeed, both the catalysts produce copolymers in which the polystyrene segments are isotactic but, as noted in the isoprene homopolymerisation, the isoprene units are arranged prevalently as trans-1,4 in the case of the catalyst 1 while in the case of catalyst 2 a higher content of cis-1,4-units and 3,4 units are present. Besides, considering a copolymer with the same monomer feeding (run 2 vs. run 8) it is evident the tendency of catalyst 2 to incorporate a major amount of isoprene. The higher amount of 3,4-units in the polymer chain also causes an increase of the Tg in the copolymers having similar composition (run 4 vs. run 11). Considering that the methyl group in para-position does not significantly affect the 13C NMR chemical shifts of the carbon atoms in the main chain the attributions of the 13C signals were performed using the previously reported data for the p-methylstyrene–isoprene copolymers.6i
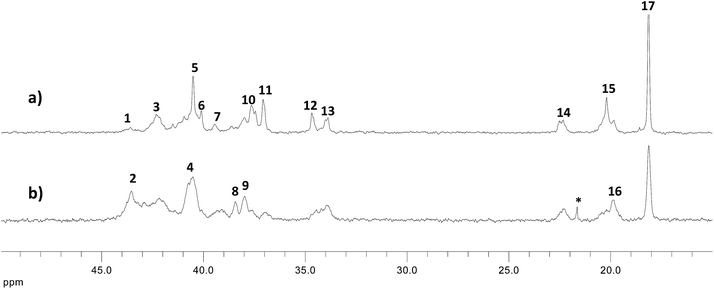
In Fig. 1 the spectra of the copolymers obtained in presence of the catalyst 1 (a, run 3) and catalyst 2 (b, run 9) having similar composition are shown. On one hand, the 13C NMR spectrum (a) of the S–I copolymers by catalyst 1 exhibit signals attributed to isotactic styrene triad SSS and TT (T = trans-1,4-isoprene) (see Scheme 2) corresponding to trans-1,4-isoprene dyad respectively. In detail the 13C NMR spectrum of the S–I copolymers by catalyst 1 showed S homosequences in isotactic arrangement with diagnostic signals for SS1S and SS2S at 43.4 and 40.9 ppm, respectively, and for the isoprene units the main signals are due to the TT1 and T4T dyads at 39.9 and 26.9 ppm. In addition, the presence of 3,4 and cis-1,4 isoprene units is only detectable from the signals relative to the methyl of the isoprene unit respectively at 18.7 ppm and 23.6 ppm denoting that these units are isolated along the polymer backbone.
 | ||
| Fig. 1 Aliphatic region of the 13C NMR spectrum of entry 3 (a, xs = 0.40) and entry 9 (b, xs = 0.36) of Table 1. | ||
On the other hand the spectrum of the copolymer obtained in the presence of the catalyst 2 clearly shows a higher amount of cis-1,4 and 3,4 isoprene units. As a matter of fact the signal relative to the methyl C5 and V5 are sensibly higher than that relative to the trans-1,4-units T5. Consequently the signals relative to the VS1V, CS2C and VS1C′ are also present. A complete assignment was made by comparison with the literature data4f,6i and the results are reported in Table 2. Finally the average styrene block lengths for copolymers obtained in the presence of catalyst 1 and 2 are ranging between 1.7–35 in the first case and between 1.3–34 in the second case revealing for both systems a relative low tendency to form long styrene homosequences.
| Peak number | Sequence | Chemical shift (observed) | Chemical shift (from literature)4f,6i |
|---|---|---|---|
| a The numbering and symbols are those indicated in Scheme 2. | |||
| 1 | VS1V | 48.1 | 48.4 |
| 2 | TS2T/CS2C | 45.7 | 45.8 |
| 3 | VS1C′ | 44.9 | 44.8 |
| 4 | TS2S | 43.6 | 43.5 |
| 5 | SS1S | 43.4 | 43.2 |
| 6 | SS2T | 42.6 | 42.5 |
| 7 | SS2S | 40.9 | 40.9 |
| 8 | TT1 | 39.9 | 40.0 |
| 9 | TST1 | 37.7 | 38.2 |
| 10 | SST1 | 37.4 | 38.2 |
| 11 | TS1T | 35.6 | 35.4 |
| 12 | SS1T | 34.8 | 35.0 |
| 13 | ST4S/T4SS | 34.1 | 34.1 |
| 14 | CC1C | 32.5 | 32.4 |
| 15 | C′C1 | 31.4 | 30.8 |
| 16 | SC1C/SC1S/VC1 | 30.0 | 30.1 |
| 17 | C4C′ | 28.4 | 28.6 |
| 18 | T4T | 26.9 | 26.9 |
| 19 | CC4C | 26.5 | 26.6 |
| 20 | C5 | 23.6 | 23.7 |
| 21 | V5 | 18.7 | 18.8 |
| 22 | T5 | 16.2 | 16.2 |
Copolymerisation of styrene with 1,3-pentadiene
As in the case of isoprene also in the case of 1,3-pentadiene the catalyst 1 and 2 were firstly tested in the homopolymerisation reactions. As already observed only the E-1,3-pentadiene is polymerised while the Z-PD is completely unreactive.6h Because of the large availability and low cost of the mixture of isomers we then used such mixture in which the E-isomer is the major component (2/1 ratio). Notably, also in this case the behaviour of the two catalysts shows some difference regarding the stereoselectivity. Indeed, the more bulky complex 2 gives a polymer containing a major amount of 1,2-units 77% vs. 65% (runs 21 and 14) confirming the tendency, observed in the case of isoprene, of more steric demanding metal complex to produce a polymer with higher vinyl content (3,4 in the case of isoprene and 1,2 in the case of 1,3-pentadine). It is worth remarking that the difference in the stereoselectivity between the catalysts 1 and 2 is less pronounced for 1,3-pentadiene with slightly preference of the more bulky catalyst 2 to give a higher content of 1,2-units. Actually, considering that there is no difference in the stereoselectivity in the case of butadiene polymerisation producing in both cases a polymer with high trans-1,4 content (≥95%),6d,7a the results obtained in the case of isoprene and 1,3-pentadiene show that both increasing the steric bulk on the metal centre and increasing the size of the monomer result in a progressive increment of the vinyl (1,2 or 3,4) content in the resulting polymer. In particular, for isoprene, that can be considered a 2-methyl substituted butadiene the 1,4-trans stereochemistry is still the preferred for the catalyst 1 (91% run 1) while for the more sterically congested catalyst 2 become important the content in the final polyisoprene of 3,4-units (29% run 7). In the case of 1,3-pentadiene the 1,2 regiochemistry is favoured over 1,4 for both catalysts (runs 14 and 21 Table 3) and become almost exclusive in the case of 4-methyl-1,3-pentadiene7b,9 clearly showing the synergic effect of the catalyst-monomer structure in determining the stereochemistry of the resulting polymers for 1,3-alkadienes for this family of catalysts. A series of styrene-1,3-pentadiene copolymers with a wide range of composition (xs = 0.10–0.85) were obtained by using the catalysts 1 and 2 activated by MAO.| Entrya | Catalyst | [S]/[PD] | Yield g (%) | Compositione (mol%) | Mw (× 103 Da) | Mw/Mn | Tg (°C) | ns | ||
|---|---|---|---|---|---|---|---|---|---|---|
| S | PDV | PDT | ||||||||
| a Polymerisation conditions: 19 μmol of complex, 3.0 × 10−2 mol of 1,3-PD, 1.31 g of MAO (Al/Ti = 1200), 15 mL of toluene, polymerisation time: 3 h, temperature: 50 °C.b 5.0 × 10−2 mol of 1,3-PD.c Polymerisation conditions: 22 μmol of complex, 46.0 × 10−2 mol of 1,3-PD, 15 mL of MAO (Al/Ti = 1000), 40 mL of toluene, polymerisation time: 1 h, temperature: 50 °C.d Same conditions of c but with 2.0 × 10−2 mol of 1,3-PD.e Determined by 1H and 13C NMR analysis; PDV = vinyl (e.g. 1,2) 1,3-PD units; PDT = trans-1,4-PD units. | ||||||||||
| 14 | 1 | 0.4 (12) | — | 65 | 35 | 94 | 1.3 | −9.7 | — | |
| 15b | 1 | 0.02 | 0.4 (11) | 10 | 62 | 28 | 90 | 1.5 | −8.6 | 2.1 |
| 16 | 1 | 0.03 | 0.9 (42) | 39 | 41 | 20 | 118 | 1.5 | −0.3 | 3.0 |
| 17 | 1 | 0.1 | 0.3 (13) | 52 | 37 | 11 | 107 | 1.5 | 6.2 | 5.7 |
| 18b | 1 | 0.5 | 5.0 (80) | 60 | 34 | 6 | 314 | 1.4 | 34.4 | 7.5 |
| 19 | 1 | 1.0 | 4.3 (95) | 71 | 22 | 7 | 364 | 1.2 | 50.6 | 9.6 |
| 20 | 1 | 2.0 | 6.8 (82) | 80 | 13 | 7 | 620 | 1.9 | 56.8 | 10.1 |
| 21 | 2 | 0.8 (13) | — | 77 | 23 | 131 | 1.1 | −7.1 | — | |
| 22c | 2 | 0.02 | 1.7 (41) | 8 | 65 | 27 | 170 | 1.2 | −15.2 | 2.8 |
| 23c | 2 | 0.03 | 2.2 (52) | 14 | 58 | 28 | 180 | 1.2 | −12.4 | 2.9 |
| 24c | 2 | 0.1 | 3.8 (80) | 27 | 52 | 21 | 210 | 1.3 | −1.4 | 3.6 |
| 25d | 2 | 0.5 | 1.8 (75) | 63 | 25 | 12 | 290 | 1.3 | 30.3 | 5.2 |
| 26d | 2 | 1.0 | 3.2 (93) | 74 | 18 | 8 | 305 | 1.4 | 47.3 | 7.9 |
| 27d | 2 | 2.0 | 3 (100) | 85 | 10 | 5 | 274 | 1.4 | 49.4 | 9.8 |
GPC analysis of these copolymers showed that the molecular weights distributions are monomodal with PDI values ranging from 1.2 to 1.9 confirming the copolymeric nature of the material. The molecular weight, in both cases, increases by increasing the styrene content and there is not an appreciable difference between the two catalytic systems. As already observed for the styrene–isoprene copolymers, also in this case the Tg values increase by increasing the styrene content ranging from −15.2 °C in the case of the copolymer containing the higher 1,3-pentadiene content (run 22) to a value of 56.8 °C for the polymer containing higher styrene content (run 20).
Remarkably, by using the same molar ratio between the two co-monomers the styrene–1,3-pentadiene copolymers contain a higher amount of the diene monomer compared to the styrene–isoprene copolymers produced with same catalytic system (run 11 Table 1 vs. run 24 Table 3) denoting a higher reactivity for this monomer respect to the isoprene.
A deeper insight into the microstructural features of the copolymers comes from the inspection of the 13CNMR spectra of the copolymers at different compositions. In Fig. 2 the spectra of the copolymer containing 14% of 1,3-pentadiene (run 23) and 63% (run 25) of 1,3-pentadiene are shown. As evident from the signals in the methyl region, the 1,3-pentadiene is mainly present as 1,2-trans and in lower amount as 1,4 showing that the copolymerisation with styrene does not affect the stereochemistry of insertion of the diene monomer respect to the homopolymerisation. Moreover the aliphatic region of the spectra clearly shows the signals due to the styrene and 1,3-pentadiene homosequences and the signals attributable to the styrene–1,3-pentadiene heterodyads.11 The complete attribution is reported in the Table 4 following the nomenclature in the Scheme 3. It is worth noting that in this case the copolymers obtained in the presence of the catalyst 1 and 2, having similar composition, do not show appreciable microstructural differences. Furthermore these new copolymers present even shorter styrene homosequences due the higher relative reactivity of the 1,3-pentadiene respect to isoprene.
 | ||
| Fig. 2 Aliphatic region of the 13C NMR spectra of entry 23 (a, xs = 0.14) and entry 25 (b, xs = 0.63) of Table 3. Peak marked with * is due to toluene. | ||
| Peak number | Sequence | Chemical shift (observed) | Chemical shift (from literature)6i,12 |
|---|---|---|---|
| a The numbering and symbols are those indicated in Scheme 3. | |||
| 1 | SS2T | 43.7 | 43.5 |
| 2 | SS1T | 43.5 | 43.4 |
| 3 | SS1S/TV1V | 42.9–42.3 | 43.2–42.6 |
| 4 | SS2S | 40.9 | 40.9 |
| 5 | VV1V | 40.5 | 40.9 |
| 6 | T4T | 40.1 | 40.3 |
| 7 | VT1 | 39.5 | 38.6 |
| 8 | T4S | 38.6 | 37.9 |
| 9 | VV2S | 38.0 | 37.7 |
| 10 | VV2V | 37.6 | 37.7 |
| 11 | TT1 | 37.1 | 36.8 |
| 12 | T4V | 34.7 | 34.5 |
| 13 | T′4T | 33.9 | 33.9 |
| 14 | T5V5 | 22.6 | 22.0 |
| 15 | T5T | 20.2 | 20.0 |
| 16 | T5T′ | 19.8 | 20.0–20.2 |
| 17 | V5V | 18.1 | 17.9 |
Conclusions
In this work we have reported the binary copolymerisation of styrene with isoprene and 1,3-pentadiene promoted by two catalytic systems based on [OSSO]-type titanium complexes activated by methylaluminoxane. Notably both catalysts are able to promote the copolymerisation of styrene with isoprene and 1,3-pentadiene giving the resulting copolymers with a wide range of compositions and in good yield. The complete characterization by GPC, DSC and 13C NMR of the resulting copolymers showed that is possible to obtain copolymers having different microstructural features by changing the catalytic system. As a matter of fact, on one hand the styrene–isoprene copolymers obtained by using the catalyst 1 show a high stereoselectivity for styrene and isoprene giving polymer chain in which the styrenic monomer homosequences are isotactic and the isoprene monomers are inserted preferentially with 1,4-trans-selectivity. On the other hand the catalyst 2 gives copolymers with isotactic styrene homosequences but the isoprene segments contain a high degree of 3,4 and 1,4-cis units. In the case of the styrene–1,3-pentadiene both catalysts give copolymers with similar microstructure. These results show not only the versatility of these catalysts in the copolymerisation reactions with styrenic and diene monomers but shed more light on the catalyst structure–reactivity relationship for the stereoselective polymerisation of diene with this class of catalysts.Acknowledgements
We gratefully acknowledge the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, Roma, Italy) for FARB 2014 and PRIN 2010-11 and Pirelli Tyre S.p.A. for financial support. Dr Patrizia Oliva and Mr Stefano Torre are acknowledged for technical assistance.Notes and references
-
(a) J. N. Henderson, Styrene Butadiene Rubbers. in Rubber Technology, Chapman & Hall, London, 3rd edn, 1995, p. 209 ff and references reported therein Search PubMed
; (b) F. S. Bates, C. V. Berney and R. E. Cohen, Macromolecules, 1983, 16, 1101 CrossRef CAS
; (c) F. S. Bates, C. V. Berney and R. E. Cohen, Polymer, 1983, 24, 519 CrossRef CAS
; (d) R. F. Storey, S. E. George and M. E. Nelson, Macromolecules, 1991, 24, 2920 CrossRef CAS
; (e) K. I. Winey, E. L. Thomas and L. J. Fetters, Macromolecules, 1992, 25, 422 CrossRef CAS
; (f) R. P. Quirk and J.-J. Ma, Polym. Int., 1991, 24, 197 CrossRef CAS PubMed
.
-
(a) G. Moad and D. H. Solomon, in The Chemistry of Free Radical Polymerization, Elsevier Science Ltd, New York, 1st edn, 1995, p. 161 ff Search PubMed
; (b) P. Tate and T. W. Bethea Butadiene Polymers in Encyclopedia of Polymer Science and Engineering, John Wiley & Sons, New York, 2nd edn, 1985; vol. 2, p. 537 ff Search PubMed
; (c) H. Hsieh and R. P. Quirk, in Anionic Polymerization Principles and Practical Applications; Marcel Dekker, New York, 1996, p. 197 Search PubMed
.
- A. Proto and C. Capacchione in Stereoselective Polymerization with Single-Site Catalysts, ed. L. S. Baugh and L. A. M. Canich, CRC Press, Boca Raton, FL, 2008, p. 447 Search PubMed
.
-
(a) A. Zambelli, P. Ammendola and A. Proto, Macromolecules, 1989, 22, 2126 CrossRef CAS
; (b) L. Oliva, P. Longo, A. Grassi, P. Ammendola and C. Pellecchia, Makromol. Chem., Rapid Commun., 1990, 11, 519 CrossRef CAS PubMed
; (c) A. Zambelli, M. Caprio, A. Grassi and D. E. Bowen, Macromol. Chem. Phys., 2000, 201, 393 CrossRef CAS
; (d) M. Caprio, M. C. Serra, D. E. Bowen and A. Grassi, Macromolecules, 2002, 35, 9315 CrossRef CAS
; (e) H. T. Ban, T. Kase, M. Kawabe, A. Miyazawa, T. Ishihara, H. Hagihara, Y. Tsunogae, M. Murata and T. Shiono, Macromolecules, 2006, 39, 171 CrossRef CAS
; (f) C. Cuomo, M. C. Serra, M. Gonzalez Maupoey and A. Grassi, Macromolecules, 2007, 40, 7089 CrossRef CAS
.
-
(a) S. Kaita, Z. Hou and Y. Wakatsuki, Macromolecules, 2001, 34, 1539 CrossRef CAS
; (b) P. Zinck, M. Terrier, A. Mortreaux, A. Valente and M. Vissaux, Macromol. Chem. Phys., 2007, 208, 973 CrossRef CAS PubMed
; (c) P. Zinck, A. Valente, A. Mortreaux and M. Vissaux, Polymer, 2007, 48, 4609 CrossRef CAS PubMed
; (d) H. Zhang, Y. J. Luo and Z. Hou, Macromolecules, 2008, 41, 1064 CrossRef CAS
; (e) A. S. Rodrigues, E. Kirillov, B. Vuillemin, A. Razavi and J. F. Carpentier, Polymer, 2008, 49, 2039 CrossRef CAS PubMed
; (f) Z. Jian, D. Cui, Z. Hou and X. Li, Chem. Commun., 2010, 46, 3022 RSC
; (g) L. Annunziata, M. Duc and J. F. Carpentier, Macromolecules, 2011, 44, 7158–7166 CrossRef CAS
; (h) A. Valente, G. Stoclet, F. Bonnet, A. Mortreux, M. Visseaux and P. Zinck, Angew. Chem., Int. Ed., 2014, 53, 4638–4641 CrossRef CAS PubMed
.
-
(a) C. Capacchione, M. D'Acunzi, O. Motta, L. Oliva, A. Proto and J. Okuda, Macromol. Chem. Phys., 2004, 205, 370–373 CrossRef CAS PubMed
; (b) C. Capacchione, A. Proto, H. Ebeling, R. Mülhaupt and J. Okuda, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1908–1913 CrossRef CAS PubMed
; (c) F. De Carlo, C. Capacchione, V. Schiavo and A. Proto, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1486–1491 CrossRef CAS PubMed
; (d) S. Milione, C. Cuomo, C. Capacchione, C. Zannoni, A. Grassi and A. Proto, Macromolecules, 2007, 40, 5638–5643 CrossRef CAS
; (e) C. Capacchione, A. Avagliano and A. Proto, Macromolecules, 2008, 41, 4573–4575 CrossRef CAS
; (f) A. Proto, A. Avagliano, D. Saviello and C. Capacchione, Macromolecules, 2009, 42, 6981–6985 CrossRef CAS
; (g) C. Capacchione, D. Saviello, A. Avagliano and A. Proto, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4200–4206 CrossRef CAS PubMed
; (h) C. Costabile, C. Capacchione, D. Saviello and A. Proto, Macromolecules, 2012, 45, 6363–6370 CrossRef CAS
; (i) A. Buonerba, M. Fienga, S. Milione, C. Cuomo, A. Grassi, A. Proto and C. Capacchione, Macromolecules, 2013, 46, 8449–8457 CrossRef CAS
.
-
(a) A. Proto, A. Avagliano, D. Saviello, R. Ricciardi and C. Capacchione, Macromolecules, 2010, 43, 5919–5921 CrossRef CAS
; (b) C. Capacchione, D. Saviello, R. Ricciardi and A. Proto, Macromolecules, 2011, 44, 7940–7947 CrossRef CAS
.
-
(a) C. Capacchione, A. Proto, H. Ebeling, R. Mülhaupt, K. Möller, T. P. Spaniol and J. Okuda, J. Am. Chem. Soc., 2003, 125, 4964–4965 CrossRef CAS PubMed
; (b) C. Capacchione, R. Manivannan, M. Barone, K. Beckerle, R. Centore, L. Oliva, A. Proto, A. Tuzi, T. P. Spaniol and J. Okuda, Organometallics, 2005, 24, 2971–2982 CrossRef CAS
.
- A. Proto, C. Capacchione, V. Venditto and J. Okuda, Macromolecules, 2003, 36, 9249–9251 CrossRef CAS
.
-
(a) S. Pragliola, G. Milano, G. Guerra and P. Longo, J. Am. Chem. Soc., 2002, 124, 3502–3503 CrossRef CAS PubMed
; (b) P. Longo, S. Pragliola, G. Milano and G. Guerra, J. Am. Chem. Soc., 2003, 125, 4799–4803 CrossRef CAS PubMed
; (c) P. Longo, M. Napoli, S. Pragliola, C. Costabile, G. Milano and G. Guerra, Macromolecules, 2003, 36, 9067–9074 CrossRef CAS
.
- The only example of styrene–1,3-pentadiene copolymerisation using syndiospecific titanium catalyst: P. Longo, A. Proto, P. Oliva, I. Sessa and A. Zambelli, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2697–2702 CrossRef CAS
.
-
(a) V. A. Rozentsvet, A. S. Khachaturov and V. P. Ivanova, Polym. Sci., Ser. A, 2006, 48, 601–605 CrossRef
; (b) D. Xie, Z. Gong and F. Wang, Chin. J. Polym. Sci., 1987, 5, 109–113 Search PubMed
; (c) A. L. Segre, M. Delfini, F. Conti and A. Boicelli, Polymer, 1975, 16, 338–344 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: 13C NMR and DEPT 135 of the copolymers of listed in Table 1 and 3. Equations for the evaluation of the average styrene block length. DSC curves of the copolymers in Table 1 and 3. See DOI: 10.1039/c5ra11866b |
| This journal is © The Royal Society of Chemistry 2015 |